Biology:WIN-35428
 | |
| Clinical data | |
|---|---|
| Other names | CFT, WIN 35,428 |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
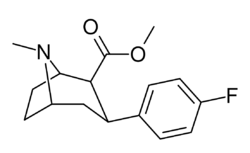
| Formula | C16H20FNO2 |
| Molar mass | 277.339 g·mol−1 |
| 3D model (JSmol) | |
| Specific rotation | -62.5° |
| Melting point | 202 to 204 °C (396 to 399 °F) |
| |
| |
| | |
(–)-2-β-Carbomethoxy-3-β-(4-fluorophenyl)tropane (β-CFT, WIN 35,428) is a stimulant drug used in scientific research. CFT is a phenyltropane based dopamine reuptake inhibitor and is structurally derived from cocaine. It is around 3-10x more potent than cocaine and lasts around 7 times longer based on animal studies. While the naphthalenedisulfonate salt is the most commonly used form in scientific research due to its high solubility in water, the free base and hydrochloride salts are known compounds and can also be produced. The tartrate is another salt form that is reported.[1]
Uses
CFT was first reported by Clarke and co-workers in 1973.[2] This drug is known to function as a "positive reinforcer" (although it is less likely to be self-administered by rhesus monkeys than cocaine).[1] Tritiated CFT is frequently used to map binding of novel ligands to the DAT, although the drug also has some SERT affinity.
Radiolabelled forms of CFT have been used in humans and animals to map the distribution of dopamine transporters in the brain. CFT was found to be particularly useful for this application as a normal fluorine atom can be substituted with the radioactive isotope 18F which is widely used in Positron emission tomography. Another radioisotope-substituted analog [11C]WIN 35,428 (where the carbon atom of either the N-methyl group, or the methyl from the 2-carbomethoxy group of CFT, has been replaced with 11C) is now more commonly used for this application, as it is quicker and easier in practice to make radiolabelled CFT by methylating nor-CFT or 2-desmethyl-CFT than by reacting methylecgonidine with parafluorophenylmagnesium bromide, and also avoids the requirement for a licence to work with the restricted precursor ecgonine.
CFT is about as addictive as cocaine in animal studies, but is taken less often due to its longer duration of action. Potentially this could make it a suitable drug to be used as a substitute for cocaine, in a similar manner to how methadone is used as a substitute for opiates in treating addiction.
Street drug
In August 2010, some media sources claimed that the designer drug Ivory Wave contained WIN 35428.[3] However, samples of Ivory Wave have been found to contain MDPV,[4] so the legitimacy of these claims remains unclear.
Legal status
CFT is not specifically scheduled in the United States,[5] though it meets the statutory definition of an ecgonine derivative. Consequently, it is a Schedule II drug.[6]
Toxicity
Administering 100 mg/kg of CFT to rats only resulted in convulsions being reported, whereas CIT had the ability to cause death at this dose.[7]
See also
- WIN 35,065-2
- List of phenyltropanes
- List of cocaine analogues
References
- ↑ 1.0 1.1 "A reduced rate of in vivo dopamine transporter binding is associated with lower relative reinforcing efficacy of stimulants". Neuropsychopharmacology 31 (2): 351–362. February 2006. doi:10.1038/sj.npp.1300795. PMID 15957006.
- ↑ "Compounds affecting the central nervous system. 4. 3 Beta-phenyltropane-2-carboxylic esters and analogs". Journal of Medicinal Chemistry 16 (11): 1260–1267. November 1973. doi:10.1021/jm00269a600. PMID 4747968.
- ↑ "Ivory Wave: The new meow meow?". Metro.co.uk. 2010-08-17. http://www.metro.co.uk/news/838315-ivory-wave-the-new-meow-meow.
- ↑ "Ivory Wave drug implicated in death of 24-year-old man | Society | guardian.co.uk". London: Guardian. 2010-08-17. https://www.theguardian.com/society/2010/aug/17/ivory-wave-drug-alleged-death.
- ↑ "DEA, Drug Scheduling". https://www.justice.gov/dea/pubs/scheduling.html.
- ↑ "21 USC 812: Schedules of controlled substances". https://uscode.house.gov/view.xhtml?req=38&f=treesort&num=5465.
Further reading
- "Conditioned taste aversion and operant behaviour in rats: effects of cocaine and a cocaine analogue (WIN 35,428)". Neuropharmacology 18 (12): 1009–1010. December 1979. doi:10.1016/0028-3908(79)90167-9. PMID 530372.
- "Saturable (3H)cocaine binding in central nervous system of mouse". Life Sciences 27 (12): 1055–1062. September 1980. doi:10.1016/0024-3205(80)90029-6. PMID 6106874.
- "Self-administration of the high-affinity cocaine analog 2 beta-carbomethoxy-3 beta-(4-fluorophenyl)tropane". Pharmacology, Biochemistry, and Behavior 39 (4): 1011–1013. August 1991. doi:10.1016/0091-3057(91)90067-c. PMID 1763097.
- "Synthesis and receptor binding of N-substituted tropane derivatives. High-affinity ligands for the cocaine receptor". Journal of Medicinal Chemistry 34 (5): 1728–1731. May 1991. doi:10.1021/jm00109a029. PMID 2033595.
- "Behavioral effects of novel cocaine analogs: a comparison with in vivo receptor binding potency". The Journal of Pharmacology and Experimental Therapeutics 260 (3): 1174–1179. March 1992. PMID 1545384.
- "Chemistry, design, and structure-activity relationship of cocaine antagonists". Chemical Reviews 100 (3): 925–1024. March 2000. doi:10.1021/cr9700538. PMID 11749256.
- "Interactions of cocaine with dopamine uptake inhibitors or dopamine releasers in rats discriminating cocaine". The Journal of Pharmacology and Experimental Therapeutics 317 (3): 1088–1096. June 2006. doi:10.1124/jpet.105.100594. PMID 16478825.
- "Synthesis of 3-arylecgonine analogues as inhibitors of cocaine binding and dopamine uptake". Journal of Medicinal Chemistry 33 (7): 2024–2027. July 1990. doi:10.1021/jm00169a036. PMID 2362282.
- "Stereoselective synthesis of 2β-carbomethoxy-3β-phenyltropane derivatives. Enhanced stereoselectivity observed for the conjugate addition reaction of phenylmagnesium bromide derivatives with anhydro dichloromethane.". Journal of Heterocyclic Chemistry 33 (6): 2037–9. November 1996. doi:10.1002/jhet.5570330676.
 |

