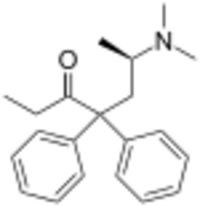
Chemistry:Levomethadone
 | |
 | |
| Clinical data | |
|---|---|
| Other names | Levamethadone; l-Methadone; 6R-Methadone; (–)-Methadone; R-(–)-Methadone; D-(–)-Methadone |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth, IV, IM, SC, IT[1] |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | High[1] |
| Protein binding | 60–90%[1] |
| Elimination half-life | ~18 hours[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H27NO |
| Molar mass | 309.453 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 99.5 °C (211.1 °F) |
| Solubility in water | 48.48 mg/mL (20 °C) |
| |
| |
Levomethadone, sold under the brand name L-Polamidon among others, is a synthetic opioid analgesic and antitussive which is marketed in Europe and is used for pain management and in opioid maintenance therapy.[1][2][3] In addition to being used as a pharmaceutical drug itself, levomethadone is the main therapeutic component of methadone.[2]
Levomethadone is used for narcotic maintenance in place of, or in some cases alongside as an alternative, to racemic methadone,[4] owing to concern about the cardiotoxic and QT-prolonging action of racemic methadone being exclusively caused by the dextrorotatory enantiomer, dextromethadone.[5][4]
Pharmacology
Pharmacodynamics
Levomethadone has approximately 50x the potency of the S-(+)-enantiomer as well as greater μ-opioid receptor selectivity.[1][6] Accordingly, it is about twice as potent as methadone by weight and its effects are virtually identical in comparison.[7][8] In addition to its activity at the opioid receptors, levomethadone has been found to act as a weak competitive antagonist of the N-methyl-D-aspartate (NMDA) receptor complex[9] and as a potent noncompetitive antagonist of the α3β4 nicotinic acetylcholine (nACh) receptor.[10]
Chemistry
The separation of the stereoisomers is one of the easier in organic chemistry and is described in the original patent.[11] It involves "treatment of racemic methadone base with d-(+)-tartaric acid in an acetone/water mixture [which] precipitates almost solely the dextro-methadone levo-tartrate, and the more potent Levomethadone can easily be retrieved from the mother liquor in a high state of optical purity."[12]
There is now an asymmetric synthesis[13] available to prepare both levomethadone (R-(−)-methadone) and dextromethadone (S-(+)-methadone).[14]
Society and culture
Generic names
Levomethadone is the generic name of the drug and its INN.[3][2]
Brand names
Levomethadone has been sold under brand names including L-Polaflux, L-Polamidon, L-Polamivet, Levadone, Levo-Methasan, Levothyl, Mevodict, Levopidon and Vistadict, among others.[15][3][2]
Legal status
Levomethadone is listed under the Single Convention On Narcotic Drugs 1961 and is a Schedule II Narcotic controlled substance in the US as an isomer of methadone (ACSCN 9250) and is not listed separately, nor is dextromethadone.[16] It is similarly controlled under the German Betäubungsmittelgesetz and similar laws in practically every other country.[17][18]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Analgesics: From Chemistry and Pharmacology to Clinical Application. Wiley-VCH. 20 December 2002. p. 196. ISBN 978-3-527-30403-5. https://books.google.com/books?id=f-nbCxYZMYkC&pg=PA196. Retrieved 17 May 2012.
- ↑ 2.0 2.1 2.2 2.3 Dictionary of Pharmacological Agents. CRC Press. 1997. p. 1294. ISBN 978-0-412-46630-4. https://books.google.com/books?id=A0THacd46ZsC&pg=PA1294. Retrieved 17 May 2012.
- ↑ 3.0 3.1 3.2 Index Nominum 2000: International Drug Directory. Taylor & Francis US. 2000. p. 605. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA605. Retrieved 17 May 2012.
- ↑ 4.0 4.1 "Side effects of levomethadone and racemic methadone in a maintenance program". Clinical Pharmacology and Therapeutics 20 (4): 445–449. October 1976. doi:10.1002/cpt1976204445. PMID 788990.
- ↑ "Levomethadone - an overview | ScienceDirect Topics". https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/levomethadone.
- ↑ Surface Design: Applications in Bioscience and Nanotechnology. Wiley-VCH. 11 August 2009. p. 193. ISBN 978-3-527-40789-7. https://books.google.com/books?id=J0fhTBYVJioC&pg=PA193. Retrieved 17 May 2012.
- ↑ Oxford American Handbook of Hospice and Palliative Medicine. Oxford University Press. 16 August 2011. p. 43. ISBN 978-0-19-538015-6. https://books.google.com/books?id=I1laHM-HP1QC&pg=PA43. Retrieved 17 May 2012.
- ↑ "The effects of racemic D,L-methadone and L-methadone in substituted patients--a randomized controlled study". Drug and Alcohol Dependence 80 (2): 267–271. November 2005. doi:10.1016/j.drugalcdep.2005.04.007. PMID 15916866.
- ↑ The Treatment of Opioid Dependence. JHU Press. 4 November 2005. p. 63. ISBN 978-0-8018-8303-3. https://books.google.com/books?id=entNMgTdeJ4C&pg=PA63. Retrieved 19 May 2012.
- ↑ "Blockade of rat alpha3beta4 nicotinic receptor function by methadone, its metabolites, and structural analogs". The Journal of Pharmacology and Experimental Therapeutics 299 (1): 366–371. October 2001. PMID 11561100. http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=11561100. Retrieved 2012-05-19.
- ↑ "The significance of chirality in drug design and development". Current Topics in Medicinal Chemistry 11 (7): 760–770. 2011. doi:10.2174/156802611795165098. PMID 21291399.
- ↑ "Synthesis of Methadone". Erowid. https://www.erowid.org/archive/rhodium/chemistry/methadone.html.
- ↑ "Synthesis of optically active methadones, LAAM and bufuralol by lipase-catalysed acylations". Tetrahedron: Asymmetry 14 (5): 567–576. March 2003. doi:10.1016/S0957-4166(03)00019-3.
- ↑ Scheinmann F, Hull JD, Turner NJ, "Process for the preparation of optically active methadones in high enantiomeric purity", US patent 6143933, issued 7 November 2000, assigned to Salford Ultrafine Chemicals.
- ↑ "Levomethadone". Drugs.com. https://www.drugs.com/international/levomethadone.html.
- ↑ "Conversion Factors for Controlled Substances". Diversion Control Division. Drug Enforcement Administration, United States Department of Justice. http://www.deadiversion.usdoj.gov/quotas/conv_factor/index.html.
- ↑ "Opioid prescription patterns in Germany and the global opioid epidemic: Systematic review of available evidence". PLOS ONE 14 (8): e0221153. 2019-08-28. doi:10.1371/journal.pone.0221153. PMID 31461466. Bibcode: 2019PLoSO..1421153R.
- ↑ "Reviewing current practice in drug-substitution treatment in the European Union". https://www.emcdda.europa.eu/system/files/publications/111/Insight3_64348.pdf.
 |

