Chemistry:Pregnanolone
| |
| Names | |
|---|---|
| IUPAC name
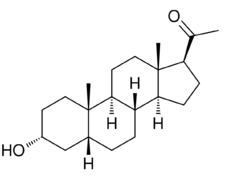
3α-Hydroxy-5β-pregnan-20-one
| |
| Systematic IUPAC name
1-[(1S,3aS,3bR,5aR,7R,9aS,9bS,11aS)-7-Hydroxy-9a,11a-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-1-yl]ethan-1-one | |
| Other names
Eltanolone; 5β-Pregnan-3α-ol-20-one; 3α,5β-Tetrahydroprogesterone; 3α,5β-THP; 3α-Hydroxy-5β-tetrahydroprogesterone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C21H34O2 | |
| Molar mass | 318.501 g·mol−1 |
| Pharmacology | |
| Intravenous injection[1] | |
| Pharmacokinetics: | |
| 0.9–3.5 hours[1][2][3] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pregnanolone, also known as eltanolone, is an endogenous inhibitory neurosteroid which is produced in the body from progesterone.[4] It is closely related to allopregnanolone, which has similar properties.[4]
Biological activity
Pregnanolone is a positive allosteric modulator of the GABAA receptor,[4] as well as a negative allosteric modulator of the glycine receptor.[5]
Biological function
Pregnanolone has sedative, anxiolytic, anesthetic, and anticonvulsant effects.[4][5][1] During pregnancy, pregnanolone and allopregnanolone are involved in sedation and anesthesia of the fetus.[6][7]
Biochemistry
Pregnanolone is synthesized from progesterone via the enzymes 5β-reductase and 3α-hydroxysteroid dehydrogenase, with 5β-dihydroprogesterone occurring as a metabolic intermediate. The elimination half-life of pregnanolone is between 0.9 and 3.5 hours.[1][2][3]
Chemistry
Pregnanolone, also known as 3α,5β-tetrahydroprogesterone (3α,5β-THP) or as 5β-pregnan-3α-ol-20-one, is a naturally occurring pregnane steroid and a derivative of progesterone. Related compounds include allopregnanolone (3α,5α-THP; brexanolone), epipregnanolone (3β,5β-THP), hydroxydione, isopregnanolone (3β,5α-THP), and renanolone.
History
Pregnanolone was first isolated from the urine of pregnant women in 1937.[1] Its anesthetic properties were first demonstrated in animals in 1957.[1]
Research
Pregnanolone was investigated for clinical use as a general anesthetic under the name eltanolone (INN), but produced unwanted side effects such as convulsions on occasion, and for this reason, was never marketed.[5][8][1]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 "Pharmacokinetics and pharmacodynamics of eltanolone (pregnanolone), a new steroid intravenous anaesthetic, in humans". Acta Anaesthesiol Scand 38 (7): 734–41. October 1994. doi:10.1111/j.1399-6576.1994.tb03987.x. PMID 7839787.
- ↑ 2.0 2.1 "Preliminary study of a pregnanolone emulsion (Kabi 2213) for i.v. induction of general anaesthesia". Br J Anaesth 68 (3): 272–6. March 1992. doi:10.1093/bja/68.3.272. PMID 1547051.
- ↑ 3.0 3.1 "Pregnanolone emulsion. A preliminary pharmacokinetic and pharmacodynamic study of a new intravenous anaesthetic agent". Anaesthesia 45 (3): 189–97. March 1990. doi:10.1111/j.1365-2044.1990.tb14683.x. PMID 2334030.
- ↑ 4.0 4.1 4.2 4.3 Reddy DS (2003). "Pharmacology of endogenous neuroactive steroids". Crit Rev Neurobiol 15 (3–4): 197–234. doi:10.1615/critrevneurobiol.v15.i34.20. PMID 15248811.
- ↑ 5.0 5.1 5.2 Jürgen Schüttler; Helmut Schwilden (8 January 2008). Modern Anesthetics. Springer Science & Business Media. pp. 278–. ISBN 978-3-540-74806-9. https://books.google.com/books?id=JpkkWhPbh2QC&pg=PA278.
- ↑ "The importance of 'awareness' for understanding fetal pain". Brain Res. Brain Res. Rev. 49 (3): 455–71. 2005. doi:10.1016/j.brainresrev.2005.01.006. PMID 16269314.
- ↑ "The emergence of human consciousness: from fetal to neonatal life". Pediatr. Res. 65 (3): 255–60. 2009. doi:10.1203/PDR.0b013e3181973b0d. PMID 19092726. https://philpapers.org/rec/LAGTEO-5. "[...] the fetus is sedated by the low oxygen tension of the fetal blood and the neurosteroid anesthetics pregnanolone and the sleep-inducing prostaglandin D2 provided by the placenta (36).".
- ↑ Norman Calvey; Norton Williams (21 January 2009). Principles and Practice of Pharmacology for Anaesthetists. John Wiley & Sons. pp. 110–. ISBN 978-1-4051-9484-6. https://books.google.com/books?id=lnRlU12Q4h8C&pg=PA110.
 |

