Chemistry:Diclofensine
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C17H17Cl2NO |
| Molar mass | 322.23 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Diclofensine (Ro 8-4650) was developed by Hoffmann-La Roche in the 1970s[1] in the search for a new antidepressant. It was found that the (S)-isomer was responsible for activity.[2] Diclofensine is a stimulant drug which acts as a triple monoamine reuptake inhibitor,[3][4] primarily inhibiting the reuptake of dopamine[5] and norepinephrine,[6] with affinities (Ki) of 16.8 nM, 15.7 nM, and 51 nM for DAT, NET, and SERT (dopamine, norepinephrine and serotonin transporters), respectively.[7] It was found to be an effective antidepressant in human trials,[8][9][10] with relatively few side effects,[11] but was ultimately dropped from clinical development, possibly due to concerns about its abuse potential.[12][13]
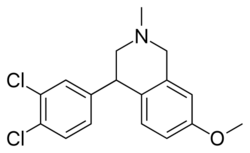
Diclofensine is chemically a tetrahydroisoquinoline (THIQ) derivative, as is nomifensine.
Synthesis
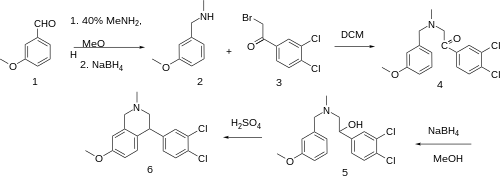
The condensation of m-anisaldehyde [591-31-1] (1) with methylamine gives N-methyl-3-methoxybenzenemethanimine [16928-30-6]. Reduction of this Schiff-base intermediate with sodium borohydride gives (3-methoxybenzyl)methylamine [41789-95-1] (2). Alkylation of this with 3,4-dichlorophenacylbromide [2632-10-2] (3) would give CID:59580342 (4). Reduction of the benzoyl ketone with sodium borohydride gives the alcohol [802051-24-7] (5). Acid catalyzed intramolecular cyclization then completes the synthesis of the 4-aryl-THIQ derivative, diclofensine (6).
See also
References
- ↑ "Substituted 4-Phenyl Isoquinolines" A US patent Patent 3947456 A, published 1976-03-30, assigned to Hoffman-La Roche Inc.
- ↑ Crossley, Roger (1995). Chirality and the Biological Activity of Drugs. 2000 Corporate Blvd., N.W., Boca Raton, Florida 33431: CRC Press, Inc.. p. 138. ISBN 978-0-8493-9140-8.
- ↑ "Diclofensine (Ro 8-4650)--a potent inhibitor of monoamine uptake: biochemical and behavioural effects in comparison with nomifensine". Advances in Biochemical Psychopharmacology 31: 249–63. 1982. PMID 6979165.
- ↑ "Pilot trials with diclofensine, a new psychoactive drug in depressed patients". International Journal of Clinical Pharmacology, Therapy, and Toxicology 20 (7): 320–6. July 1982. PMID 7107085.
- ↑ "Pure uptake blockers of dopamine can reduce prolactin secretion: studies with diclofensine". Life Sciences 42 (21): 2161–9. 1988. doi:10.1016/0024-3205(88)90131-2. PMID 2968488.
- ↑ "Effect of diclofensine, a novel antidepressant, on peripheral adrenergic function". Clinical Pharmacology and Therapeutics 39 (5): 582–5. May 1986. doi:10.1038/clpt.1986.100. PMID 3698467.
- ↑ "The dopamine inhibitor GBR 12909: selectivity and molecular mechanism of action". European Journal of Pharmacology 166 (3): 493–504. August 1989. doi:10.1016/0014-2999(89)90363-4. PMID 2530094.
- ↑ "A controlled trial with diclofensine, a new psychoactive drug, in the treatment of depression". The Journal of International Medical Research 9 (5): 324–9. 1981. doi:10.1177/030006058100900505. PMID 7028532.
- ↑ "Therapeutic efficacy and tolerance of diclofensine in psychoreactive depression--a double-blind comparison with placebo". Methods and Findings in Experimental and Clinical Pharmacology 6 (3): 147–51. March 1984. PMID 6379345.
- ↑ "Double-blind comparison of diclofensine with nomifensine in outpatients with dysphoric mood". Pharmacopsychiatry 19 (3): 120–3. May 1986. doi:10.1055/s-2007-1017168. PMID 3725890.
- ↑ "A clinical pharmacological comparison of diclofensine (Ro 8-4650) with nomifensine and amitriptyline in normal human volunteers". British Journal of Clinical Pharmacology 15 (5): 537–43. May 1983. doi:10.1111/j.1365-2125.1983.tb02087.x. PMID 6860528.
- ↑ "Self-administration in baboons and the discriminative stimulus effects in rats of bupropion, nomifensine, diclofensine and imipramine". Psychopharmacology 102 (2): 183–90. 1990. doi:10.1007/bf02245920. PMID 2125734.
- ↑ "Effects of various dopamine uptake inhibitors on striatal extracellular dopamine levels and behaviours in rats". European Journal of Pharmacology 281 (2): 195–203. August 1995. doi:10.1016/0014-2999(95)00246-h. PMID 7589207.
- ↑ Rheiner Alfred Jr Dr, CH patent 538477 (1973 to Hoffmann La Roche).
- ↑ Alfred Rheiner, U.S. Patent 3,947,456 (1976 to Hoffman-La Roche Inc.).
- ↑ Shuang Liu, Bruce F. Molino, Kassoum Nacro, WO patent 2010132442 (2010 to Albany Molecular Reserch, Inc.). Page column 32.
 |