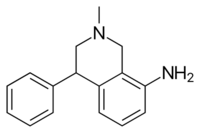
Chemistry:Nomifensine
 | |
| Clinical data | |
|---|---|
| Trade names | Merital |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 1.5–4 hours |
| Excretion | Kidney (88%) within 24 hours[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C16H18N2 |
| Molar mass | 238.334 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Nomifensine (Merital, Alival) is a norepinephrine-dopamine reuptake inhibitor, i.e. a drug that increases the amount of synaptic norepinephrine and dopamine available to receptors by blocking the dopamine and norepinephrine reuptake transporters.[2] This is a mechanism of action shared by some recreational drugs like cocaine and the medication tametraline (see DRI). Research showed that the (S)-isomer is responsible for activity.[3]
The drug was developed in the 1960s by Hoechst AG (now Sanofi-Aventis),[4] who then test marketed it in the United States . It was an effective antidepressant, without sedative effects. Nomifensine did not interact significantly with alcohol and lacked anticholinergic effects. No withdrawal symptoms were seen after 6 months treatment. The drug was however considered not suitable for agitated patients as it presumably made agitation worse.[5][6] In January 1986 the drug was withdrawn by its manufacturers for safety reasons.[7]
Some case reports in the 1980s suggested that there was potential for psychological dependence on nomifensine, typically in patients with a history of stimulant addiction, or when the drug was used in very high doses (400–600 mg per day).[8]
In a 1989 study it was investigated for use in treating adult ADHD and proven effective.[9] In a 1977 study it was not proven of benefit in advanced parkinsonism, except for depression associated with the parkinsonism.[10]
Clinical uses
Nomifensine was investigated for use as an antidepressant in the 1970s, and was found to be a useful antidepressant at doses of 50–225 mg per day, both motivating and anxiolytic.
Side effects and withdrawal from market
During treatment with nomifensine there were relatively few adverse effects, mainly renal failure, paranoid symptoms, drowsiness or insomnia, headache, and dry mouth. Side effects affecting the cardiovascular system included tachycardia and palpitations, but nomifensine was significantly less cardiotoxic than the standard tricyclic antidepressants.[11]
Due to a risk of haemolytic anaemia, the U.S. Food and Drug Administration (FDA) withdrew approval for nomifensine on March 20, 1992. Nomifensine was subsequently withdrawn from the Canadian and UK markets as well.[12] Some deaths were linked to immunohaemolytic anemia caused by this compound, although the mechanism remained unclear.[13]
In 2012 structure-affinity relationship data (compare SAR) were published.[14]
Synthesis
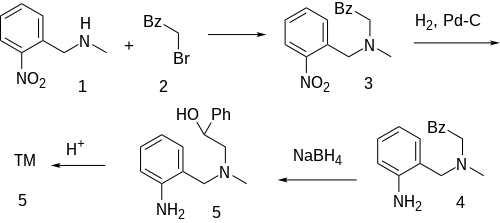
Note that nomifensine was a Progenitor to Gastrophenazine.[15] See also: Isatin derivatives.[16]
The alkylation between N-methyl-2-nitrobenzylamine [56222-08-3] (1) and phenacyl bromide (2) gives CID:15326127 (3). Catalytic hydrogenation over Raney Nickel reduces the nitro group to give CID:15113381 (4). The reduction of the ketone group with sodium borohydride to alcohol gives [65514-97-8] (5). Acid catalysed ring closure completes the formation of nomifensine (6).
See also
- Amineptine
- Diclofensine
- Perafensine
- The combination of Clobazam / nomifensine is called Psyton [75963-47-2].[26]
References
- ↑ "Kinetics and metabolism of nomifensine". The Journal of Clinical Psychiatry 45 (4 Pt 2): 21–5. April 1984. PMID 6370971.
- ↑ "Nomifensine: A review of its pharmacological properties and therapeutic efficacy in depressive illness". Drugs 18 (1): 1–24. July 1979. doi:10.2165/00003495-197918010-00001. PMID 477572.
- ↑ 'Chirality and Biological Activity of Drugs' page 138
- ↑ Ehrhart, Gustav; Karl Schmitt & Irmgard Hoffmann et al., "4-Phenyl-8-Amino Tetrahydroisoquinolines", US patent 3577424, issued 1971-05-04, assigned to Farbwerke Hoechst
- ↑ "A review of controlled studies with nomifensine, performed outside the UK". British Journal of Clinical Pharmacology 4Suppl 2 (Suppl 2): 237S–241S. 1977. doi:10.1111/j.1365-2125.1977.tb05759.x. PMID 334230.
- ↑ "An overview of side effects and long-term experience with nomifensine from United States clinical trials". The Journal of Clinical Psychiatry 45 (4 Pt 2): 96–101. April 1984. PMID 6370985.
- ↑ "CSM Update: Withdrawal of nomifensine". British Medical Journal 293 (6538): 41. July 1986. doi:10.1136/bmj.293.6538.41. PMID 20742679.
- ↑ "Nomifensine and psychological dependence--a case report". Pharmacopsychiatry 19 (5): 386–8. September 1986. doi:10.1055/s-2007-1017275. PMID 3774872.
- ↑ "Nomifensine maleate in adult attention deficit disorder". The Journal of Nervous and Mental Disease 177 (5): 296–9. May 1989. doi:10.1097/00005053-198905000-00008. PMID 2651559.
- ↑ "Nomifensine in Parkinson's disease". British Journal of Clinical Pharmacology 4Suppl 2 (Suppl 2): 187S–190S. 1977. doi:10.1111/j.1365-2125.1977.tb05751.x. PMID 334223.
- ↑ "A profile of nomifensine". British Journal of Clinical Pharmacology 4Suppl 2 (Suppl 2): 243S–248S. 1977. doi:10.1111/j.1365-2125.1977.tb05760.x. PMID 911653.
- ↑ "Nomifensine DB04821". Drugbank.ca. http://www.drugbank.ca/drugs/DB04821.
- ↑ "[Hematologic toxicity of antidepressive agents]" (in fr). L'Encéphale 14 (4): 307–18. 1988. PMID 3058454.
- ↑ "4-Phenyl tetrahydroisoquinolines as dual norepinephrine and dopamine reuptake inhibitors". Bioorganic & Medicinal Chemistry Letters 22 (23): 7219–22. December 2012. doi:10.1016/j.bmcl.2012.09.050. PMID 23084899.
- ↑ 15.0 15.1 "Synthesis and pharmacological evaluation of some new tetrahydroisoquinoline derivatives inhibiting dopamine uptake and/or possessing a dopaminomimetic property". Journal of Medicinal Chemistry 29 (7): 1189–95. July 1986. doi:10.1021/jm00157a012. PMID 3806569.
- ↑ DE3333994 idem Karl-Heinz Boltze, et al. U.S. Patent 4,564,613 (1986 to TROPONWERKE & Co KG A CORP OF GERMANY GmbH, Troponwerke GmbH).
- ↑ "[8-amino-4-phenyl-1,2,3,4-tetrahydroisoquinolines, a new group of antidepressive psycholeptic drugs]". Arzneimittel-Forschung 21 (7): 1045. July 1971. PMID 5109496.
- ↑ "Tetrahydroisoquinolines and process for preparing them" GB patent 1164192, published 1969-09-17 corresp to G. Ehrhart et al., U.S. Patent 3,577,424 (1969, 1971, both to Hoechst AG
- ↑ Gustav Dipl Chem Dr Ehrhart, et al. DE patent 1795829 (1977 to Hoechst AG).
- ↑ Orchideja Bontscheva Sabunova, et al. EP patent 0066885 (1982 to Dso "pharmachim").
- ↑ Ulin, J.; Gee, A.D.; Malmborg, P.; Tedroff, J.; Långström, B. (1989). "Synthesis of racemic (+) and (−) N-[methyl-11C]nomifensine, a ligand for evaluation of monoamine re-uptake sites by use of positron emission tomography". International Journal of Radiation Applications and Instrumentation. Part A. Applied Radiation and Isotopes. 40 (2): 171–176. doi:10.1016/0883-2889(89)90194-9.
- ↑ Ivanov, T. B.; Mondeshka, Diana M.; Angelova, Ivanka G. (1989). "Verbesserte Synthese von 8-Amino-2-methyl-4-phenyl-1,2,3,4-Tetrahydroisochinolin". Journal für Praktische Chemie. 331 (5): 731–735. doi:10.1002/prac.19893310505.
- ↑ Venkov, Atanas P.; Vodenicharov, Daniel M. (1990). "A New Synthesis of 1,2,3,4-Tetrahydro-2-methyl-4-phenylisoquinolines". Synthesis. 1990 (03): 253–255. doi:10.1055/s-1990-26846.
- ↑ Pechulis, Anthony D.; Beck, James P.; Curry, Matt A.; Wolf, Mark A.; Harms, Arthur E.; Xi, Ning; Opalka, Chet; Sweet, Mark P.; Yang, Zhicai; Vellekoop, A. Samuel; Klos, Andrew M.; Crocker, Peter J.; Hassler, Carla; Laws, Mia; Kitchen, Douglas B.; Smith, Mark A.; Olson, Richard E.; Liu, Shuang; Molino, Bruce F. (2012). "4-Phenyl tetrahydroisoquinolines as dual norepinephrine and dopamine reuptake inhibitors". Bioorganic & Medicinal Chemistry Letters. 22 (23): 7219–7222. doi:10.1016/j.bmcl.2012.09.050.
- ↑ Kunstmann, Rudolf; Gerhards, Hermann; Kruse, Hansjoerg; Leven, Margret; Paulus, Erich F.; Schacht, Ulrich; Schmitt, Karl; Witte, Peter U. (1987). "Resolution, absolute stereochemistry, and enantioselective activity of nomifensine and hexahydro-1H-indeno[1,2-b]pyridines". Journal of Medicinal Chemistry. 30 (5): 798–804. doi:10.1021/jm00388a009.
- ↑ Jellinger K, Koeppen D, Rössner M. Langzeitbehandlung depressiver Syndrome mit Psyton [Long-term treatment of depressive syndromes with Psyton (author's transl)]. Wien Med Wochenschr. 1982 Apr 30;132(8):183-8. German. PMID 6125057.
 |