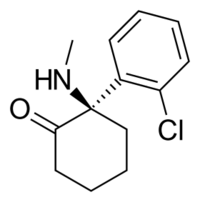
Chemistry:Arketamine
 | |
 | |
| Clinical data | |
|---|---|
| Other names | PCN-101; HR-071603 |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C13H16ClNO |
| Molar mass | 237.73 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Arketamine (developmental code names PCN-101, HR-071603), also known as (R)-ketamine or (R)-(−)-ketamine, is the (R)-(−) enantiomer of ketamine.[1][2][3] Similarly to racemic ketamine and esketamine, the S(+) enantiomer of ketamine, arketamine is biologically active; however, it is less potent as an NMDA receptor antagonist and anesthetic and thus has never been approved or marketed for clinical use as an enantiopure drug.[1][3] Arketamine is currently in clinical development as a novel antidepressant.[4][5]
Relative to esketamine, arketamine possesses 4 to 5 times lower affinity for the PCP site of the NMDA receptor.[2][6] In accordance, arketamine is significantly less potent than racemic ketamine and especially esketamine in terms of anesthetic, analgesic, and sedative-hypnotic effects.[6] Racemic ketamine has weak affinity for the sigma receptor, where it acts as an agonist, whereas esketamine binds negligibly to this receptor, and so the sigma receptor activity of racemic ketamine lies in arketamine.[7] It was suggested that this action of arketamine may play a role in the hallucinogenic effects of racemic ketamine and that it may be responsible for the lowering of the seizure threshold seen with racemic ketamine.[7] However several subsequent studies have indicated that esketamine is more likely to induce dissociative events,[8][9] while studies in patients undergoing electroconvulsive therapy suggested that esketamine is a potent inducer of seizures.[10] Esketamine inhibits the dopamine transporter about 8-fold more potently than does arketamine, and so is about 8 times more potent as a dopamine reuptake inhibitor.[11] Arketamine and esketamine possess similar potency for interaction with the muscarinic acetylcholine receptors.[12]
Novel antidepressant
Arketamine appears to be more effective as a rapid-acting antidepressant than esketamine in preclinical research.[13]
In rodent studies, esketamine produced hyperlocomotion, prepulse inhibition deficits, and rewarding effects, while arketamine did not, in accordance with its lower potency as an NMDA receptor antagonist and dopamine reuptake inhibitor.[14] As such, arketamine may have a lower propensity for producing psychotomimetic effects and a lower abuse potential in addition to superior antidepressant efficacy.[14]
A study conducted in mice found that ketamine's antidepressant activity is not caused by ketamine inhibiting NMDAR, but rather by sustained activation of a different glutamate receptor, the AMPA receptor, by a metabolite, (2R,6R)-hydroxynorketamine; as of 2017 it was unknown if this was happening in humans.[15][16] Arketamine is an AMPA receptor agonist.[17]
Paradoxically, arketamine shows greater and longer-lasting rapid antidepressant effects in animal models of depression relative to esketamine.[13][18][14] It has been suggested that this may be due to the possibility of different activities of arketamine and esketamine and their respective metabolites at the α7-nicotinic receptor, as norketamine and hydroxynorketamine are potent antagonists of this receptor and markers of potential rapid antidepressant effects (specifically, increased mammalian target of rapamycin function) correlate closely with their affinity for it.[19][20][21] The picture is unclear however, and other mechanisms have also been implicated.[14]
Clinical development
As of November 2019, arketamine is under development for the treatment of depression under the developmental code names PCN-101 by Perception Neuroscience in the United States and HR-071603 by Jiangsu Hengrui Medicine in China .[4][5]
See also
References
- ↑ 1.0 1.1 Dictionary of Pharmacological Agents. CRC Press. 21 November 1996. pp. 1188–. ISBN 978-0-412-46630-4. https://books.google.com/books?id=A0THacd46ZsC&pg=PA1188.
- ↑ 2.0 2.1 Ketamine: Use and Abuse. Taylor & Francis. 6 March 2015. pp. 269–. ISBN 978-1-4665-8340-5. https://books.google.com/books?id=Z3gZBwAAQBAJ&pg=PA269.
- ↑ 3.0 3.1 "Intravenous Esketamine in Adult Treatment-Resistant Depression: A Double-Blind, Double-Randomization, Placebo-Controlled Study". Biological Psychiatry 80 (6): 424–431. September 2016. doi:10.1016/j.biopsych.2015.10.018. PMID 26707087.
- ↑ 4.0 4.1 "Rapid-acting antidepressant ketamine, its metabolites and other candidates: A historical overview and future perspective". Psychiatry and Clinical Neurosciences 73 (10): 613–627. October 2019. doi:10.1111/pcn.12902. PMID 31215725.
- ↑ 5.0 5.1 "Arketamine - Jiangsu Hengrui Medicine". AdisInsight. Springer Nature Switzerland AG. https://adisinsight.springer.com/drugs/800056158.
- ↑ 6.0 6.1 Clinical Anesthesia. Lippincott Williams & Wilkins. 28 March 2012. pp. 456–. ISBN 978-1-4511-4795-7. https://books.google.com/books?id=wbxNC_mNuc4C&pg=PA456.
- ↑ 7.0 7.1 Drug Abuse and Addiction in Medical Illness: Causes, Consequences and Treatment. Springer Science & Business Media. 6 July 2012. pp. 205–. ISBN 978-1-4614-3375-0. https://books.google.com/books?id=yGeBj9U6Za8C&pg=PA205.
- ↑ "Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET)". European Neuropsychopharmacology 7 (1): 25–38. February 1997. doi:10.1016/S0924-977X(96)00042-9. PMID 9088882.
- ↑ "[Recovery and psychomimetic reactions following S-(+)-ketamine]". Der Anaesthesist 46 (Suppl 1): S38–S42. March 1997. doi:10.1007/pl00002463. PMID 9163277.
- ↑ "S -ketamine compared to etomidate during electroconvulsive therapy in major depression". European Archives of Psychiatry and Clinical Neuroscience 267 (8): 803–813. December 2017. doi:10.1007/s00406-017-0800-3. PMID 28424861.
- ↑ "Ketamine stereoselectively inhibits rat dopamine transporter". Neuroscience Letters 274 (2): 131–134. October 1999. doi:10.1016/s0304-3940(99)00688-6. PMID 10553955.
- ↑ Advances in Modelling and Clinical Application of Intravenous Anaesthesia. Springer Science & Business Media. 6 December 2012. pp. 270–. ISBN 978-1-4419-9192-8. https://books.google.com/books?id=dNHiBwAAQBAJ&pg=PA270.
- ↑ 13.0 13.1 "R (-)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine". Pharmacology, Biochemistry, and Behavior 116: 137–141. January 2014. doi:10.1016/j.pbb.2013.11.033. PMID 24316345.
- ↑ 14.0 14.1 14.2 14.3 "R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects". Translational Psychiatry 5 (9): e632. September 2015. doi:10.1038/tp.2015.136. PMID 26327690.
- ↑ "Classics in Chemical Neuroscience: Ketamine". ACS Chemical Neuroscience 8 (6): 1122–1134. June 2017. doi:10.1021/acschemneuro.7b00074. PMID 28418641.
- ↑ "NMDAR inhibition-independent antidepressant actions of ketamine metabolites". Nature 533 (7604): 481–486. May 2016. doi:10.1038/nature17998. PMID 27144355. Bibcode: 2016Natur.533..481Z.
- ↑ "A bright future of researching AMPA receptor agonists for depression treatment". Expert Opinion on Investigational Drugs 21 (5): 583–585. May 2012. doi:10.1517/13543784.2012.667399. PMID 22375566.
- ↑ "The R-Stereoisomer of Ketamine as an Alternative for Ketamine for Treatment-resistant Major Depression". Clinical Psychopharmacology and Neuroscience 12 (1): 72–73. April 2014. doi:10.9758/cpn.2014.12.1.72. PMID 24851126.
- ↑ "Ketamine metabolomics in the treatment of major depression". Anesthesiology 121 (1): 4–5. July 2014. doi:10.1097/ALN.0000000000000286. PMID 24936919.
- ↑ "(R,S)-Ketamine metabolites (R,S)-norketamine and (2S,6S)-hydroxynorketamine increase the mammalian target of rapamycin function". Anesthesiology 121 (1): 149–159. July 2014. doi:10.1097/ALN.0000000000000285. PMID 24936922.
- ↑ "What is hydroxynorketamine and what can it bring to neurotherapeutics?". Expert Review of Neurotherapeutics 14 (11): 1239–1242. November 2014. doi:10.1586/14737175.2014.971760. PMID 25331415.
 |

