(diff) ← Older revision | Latest revision (diff) | Newer revision → (diff)
Short description
Sulforidazine Clinical data ATC code Identifiers
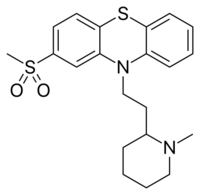
10-{2-[(RS) -1-Methylpiperidin-2-yl]ethyl}-2-(methylsulfonyl)-10H -phenothiazine
CAS Number PubChem CID ChemSpider UNII ChEBI ChEMBL Chemical and physical data Formula C 21 H 26 N 2 O 2 S 2 Molar mass −1 3D model (JSmol )
O=S(=O)(c2cc1N(c3c(Sc1cc2)cccc3)CCC4N(C)CCCC4)C
InChI=1S/C21H26N2O2S2/c1-22-13-6-5-7-16(22)12-14-23-18-8-3-4-9-20(18)26-21-11-10-17(15-19(21)23)27(2,24)25/h3-4,8-11,15-16H,5-7,12-14H2,1-2H3
Y Key:FLGCRGJDQJIJAW-UHFFFAOYSA-N
Y N Y (what is this?) (verify)
Sulforidazine (Imagotan , Psychoson , Inofal ) a typical antipsychotic and a metabolite of thioridazine ; it and mesoridazine are more potent than the parent compound, whose pharmacological effects are believed by some to be largely due to its metabolism into sulforidazine and mesoridazine.[1]
Synthesis 2-bromo-2'-amino-4'-methylsulphonyl-diphenyl Sulphide, CID:43448246 (1 )
2-bromo-2'-acetamino-4'-methylsulphonyl diphenylsulphide (2 )
2-(2-Chloroethyl)-1-Methylpiperidine [50846-01-0] (3 )
References
↑ "Greater potency of mesoridazine and sulforidazine compared with the parent compound, thioridazine, on striatal dopamine autoreceptors". Journal of Pharmacology and Experimental Therapeutics 228 (3): 636–9. March 1984. PMID 6707914 . ↑ Morrow, Ryan J.; Millership, Jeff S.; Collier, Paul S. (2005). "Facile Syntheses of the Three Major Metabolites of Thioridazine". Helvetica Chimica Acta. 88 (5): 962–967. doi:10.1002/hlca.200590089.
↑ FR1363683 idem Bruschweiler Conrad, Schwarb Gustav, Winkler Hans, Renz Jany, U.S. Patent 3,314,948
mAChRs
Agonists Antagonists
3-Quinuclidinyl benzilate 4-DAMP Aclidinium bromide (+formoterol )Abediterol AF-DX 250 AF-DX 384 Ambutonium bromide Anisodamine Anisodine Antihistamines (first-generation) (e.g., brompheniramine , buclizine , captodiame , chlorphenamine (chlorpheniramine) , cinnarizine , clemastine , cyproheptadine , dimenhydrinate , [[Chemistry:Dimetdimetindene, Diphenhydramine |diphenhydramine]], doxylamine , meclizine , mepyramine (pyrilamine) , mequitazine , perlapine , phenindamine , pheniramine , Phenyltoloxamine|Phenyltoloxamine ]]]], promethazine , propiomazine , triprolidine )AQ-RA 741 Atropine Atropine methonitrate Atypical antipsychotics (e.g., clozapine, Chemistry :Fluperlapine
Precursors (and prodrugs)
nAChRs
Agonists PAMs
5-HIAA A-84,543 A-366,833 A-582,941 A-867,744 ABT-202 ABT-418 ABT-560 ABT-894 Acetylcholine Altinicline Anabasine Anatoxin-a AR-R17779 Bephenium hydroxynaphthoate Butinoline Butyrylcholine Carbachol Choline Cotinine Cytisine Decamethonium Desformylflustrabromine Dianicline Dimethylphenylpiperazinium Epibatidine Epiboxidine Ethanol (alcohol) Ethoxysebacylcholine EVP-4473 EVP-6124 Galantamine GTS-21 Ispronicline Ivermectin JNJ-39393406 Levamisole Lobeline MEM-63,908 (RG-3487) Morantel Nicotine (tobacco )NS-1738 PHA-543,613 PHA-709,829 PNU-120,596 PNU-282,987 Pozanicline Pyrantel Rivanicline RJR-2429 Sazetidine A SB-206553 Sebacylcholine SIB-1508Y SIB-1553A SSR-180,711 Suberyldicholine Suxamethonium (succinylcholine) Suxethonium (succinyldicholine) TC-1698 TC-1734 TC-1827 TC-2216 TC-5214 TC-5619 TC-6683 Tebanicline Tribendimidine Tropisetron UB-165 Varenicline WAY-317,538 XY-4083 Antagonists NAMs
Precursors (and prodrugs)
Original source: https://en.wikipedia.org/wiki/Sulforidazine. Read more





 (0 votes)
(0 votes)
