Biology:TCF3
 Generic protein structure example |
Transcription factor 3 (E2A immunoglobulin enhancer-binding factors E12/E47), also known as TCF3, is a protein that in humans is encoded by the TCF3 gene.[1][2][3] TCF3 has been shown to directly enhance Hes1 (a well-known target of Notch signaling) expression.[4]
Function
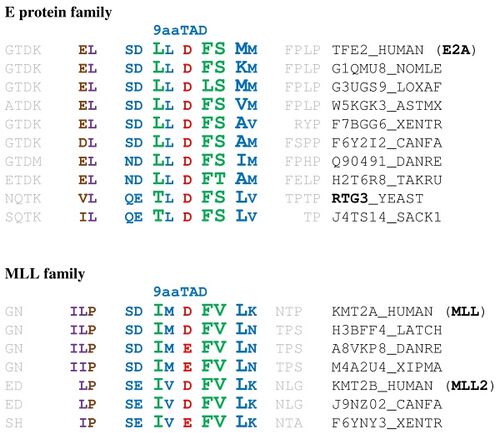
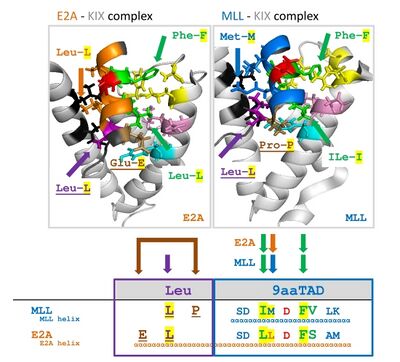
This gene encodes a member of the E protein (class I) family of helix-loop-helix transcription factors. The 9aaTAD transactivation domains of E proteins and MLL are very similar and both bind to the KIX domain of general transcriptional mediator CBP.[5][6] E proteins activate transcription by binding to regulatory E-box sequences on target genes as heterodimers or homodimers, and are inhibited by heterodimerization with inhibitor of DNA-binding (class IV) helix-loop-helix proteins. E proteins play a critical role in lymphopoiesis, and the encoded protein is required for the B and T lymphocyte development.[1]
This gene regulates many developmental patterning processes such as lymphocyte and central nervous system (CNS) development. E proteins are involved in the development of lymphocytes.[7] They initiate transcription by binding to regulatory E-box sequences on target genes.
Clinical significance
Deletion of this gene or diminished activity of the encoded protein may play a role in lymphoid malignancies. This gene is also involved in several chromosomal translocations that are associated with lymphoid malignancies including pre-B-cell acute lymphoblastic leukemia (t(1;19), with PBX1 and t(17;19), with HLF),[8] childhood leukemia (t(19;19), with TFPT) and acute leukemia (t(12;19), with ZNF384).[1]
Interactions
TCF3 has been shown to interact with:
- CBFA2T3,[9]
- CREBBP,[10]
- ELK3,[11]
- EP300,[10]
- ID3,[12][13]
- LDB1,[9]
- LMX1A,[14]
- LYL1,[15]
- MAPKAPK3,[16]
- MyoD,[13][17]
- Myogenin,[13][18]
- PCAF,[10]
- TAL1[9][19]
- TWIST1,[20] and
- UBE2I.[21]
References
- ↑ 1.0 1.1 1.2 "Entrez Gene: TCF3". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=6929.
- ↑ "Sequence of the cDNA encoding ITF-1, a positive-acting transcription factor". Nucleic Acids Research 18 (3): 677. Feb 1990. doi:10.1093/nar/18.3.677. PMID 2308859.
- ↑ "A new homeobox gene contributes the DNA binding domain of the t(1;19) translocation protein in pre-B ALL". Cell 60 (4): 547–55. Feb 1990. doi:10.1016/0092-8674(90)90658-2. PMID 1967983.
- ↑ E proteins and Notch signaling cooperate to promote T cell lineage specification and commitment
- ↑ Piskacek, S (2007). "Nine-amino-acid transactivation domain: Establishment and prediction utilities". Genomics 89 (6): 756–768. doi:10.1016/j.ygeno.2007.02.003. PMID 17467953.
- ↑ Piskacek, Martin; Vasku, A; Hajek, R; Knight, A (2015). "Shared structural features of the 9aaTAD family in complex with CBP.". Mol. Biosyst. 11 (3): 844–851. doi:10.1039/c4mb00672k. PMID 25564305.
- ↑ "E protein function in lymphocyte development". Annual Review of Immunology 20: 301–22. 2002. doi:10.1146/annurev.immunol.20.092501.162048. PMID 11861605.
- ↑ Herblot, Sabine; Aplan, Peter D.; Hoang, Trang (2002-02-01). "Gradient of E2A Activity in B-Cell Development". Molecular and Cellular Biology 22 (3): 886–900. doi:10.1128/MCB.22.3.886-900.2002. ISSN 0270-7306. PMID 11784864.
- ↑ 9.0 9.1 9.2 "ETO2 coordinates cellular proliferation and differentiation during erythropoiesis". The EMBO Journal 25 (2): 357–66. Jan 2006. doi:10.1038/sj.emboj.7600934. PMID 16407974.
- ↑ 10.0 10.1 10.2 "Regulation of E2A activities by histone acetyltransferases in B lymphocyte development". The Journal of Biological Chemistry 278 (4): 2370–6. Jan 2003. doi:10.1074/jbc.M211464200. PMID 12435739.
- ↑ "Net (ERP/SAP2) one of the Ras-inducible TCFs, has a novel inhibitory domain with resemblance to the helix-loop-helix motif". The EMBO Journal 15 (21): 5849–65. Nov 1996. doi:10.1002/j.1460-2075.1996.tb00972.x. PMID 8918463.
- ↑ "Lymphoid-specific expression of the Id3 gene in hematopoietic cells. Selective antagonism of E2A basic helix-loop-helix protein associated with Id3-induced differentiation of erythroleukemia cells". The Journal of Biological Chemistry 273 (14): 8278–86. Apr 1998. doi:10.1074/jbc.273.14.8278. PMID 9525934.
- ↑ 13.0 13.1 13.2 "Differential interactions of Id proteins with basic-helix-loop-helix transcription factors". The Journal of Biological Chemistry 272 (32): 19785–93. Aug 1997. doi:10.1074/jbc.272.32.19785. PMID 9242638.
- ↑ "Transcriptional synergy between LIM-homeodomain proteins and basic helix-loop-helix proteins: the LIM2 domain determines specificity". Molecular and Cellular Biology 17 (7): 3488–96. Jul 1997. doi:10.1128/mcb.17.7.3488. PMID 9199284.
- ↑ "Helix-loop-helix proteins LYL1 and E2a form heterodimeric complexes with distinctive DNA-binding properties in hematolymphoid cells". Molecular and Cellular Biology 16 (5): 2394–401. May 1996. doi:10.1128/mcb.16.5.2394. PMID 8628307.
- ↑ "Serine/Threonine kinases 3pK and MAPK-activated protein kinase 2 interact with the basic helix-loop-helix transcription factor E47 and repress its transcriptional activity". The Journal of Biological Chemistry 275 (27): 20239–42. Jul 2000. doi:10.1074/jbc.C901040199. PMID 10781029.
- ↑ "MyoD-E12 heterodimers and MyoD-MyoD homodimers are equally stable". Biochemistry 36 (22): 6762–7. Jun 1997. doi:10.1021/bi970262m. PMID 9184158.
- ↑ "Analysis of the oligomerization of myogenin and E2A products in vivo using a two-hybrid assay system". The Journal of Biological Chemistry 267 (25): 17498–501. Sep 1992. doi:10.1016/S0021-9258(19)37069-3. PMID 1325437.
- ↑ "Formation of in vivo complexes between the TAL1 and E2A polypeptides of leukemic T cells". Proceedings of the National Academy of Sciences of the United States of America 91 (8): 3181–5. Apr 1994. doi:10.1073/pnas.91.8.3181. PMID 8159721. Bibcode: 1994PNAS...91.3181H.
- ↑ "Saethre-Chotzen mutations cause TWIST protein degradation or impaired nuclear location". Human Molecular Genetics 9 (5): 813–9. Mar 2000. doi:10.1093/hmg/9.5.813. PMID 10749989.
- ↑ "Characterization of the mUBC9-binding sites required for E2A protein degradation". The Journal of Biological Chemistry 274 (40): 28690–6. Oct 1999. doi:10.1074/jbc.274.40.28690. PMID 10497239.
Further reading
- "E2A basic helix-loop-helix transcription factors in human leukemia". Frontiers in Bioscience 8 (1–3): s206–22. May 2003. doi:10.2741/1030. PMID 12700034.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
 |