Biology:Rel homology domain
| Rel homology domain (RHD) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||
| Identifiers | |||||||||||
| Symbol | RHD | ||||||||||
| Pfam | PF00554 | ||||||||||
| InterPro | IPR011539 | ||||||||||
| PROSITE | PDOC00924 | ||||||||||
| SCOP2 | 1svc / SCOPe / SUPFAM | ||||||||||
| CDD | cd07827 | ||||||||||
| |||||||||||
The Rel homology domain (RHD) is a protein domain found in a family of eukaryotic transcription factors,[1] including both NF-κB and NFAT, among others. Some of these transcription factors appear to form multi-protein DNA-bound complexes.[2] Phosphorylation of the RHD appears to play a role in the regulation of some of these transcription factors, acting to modulate the expression of their target genes.[3]
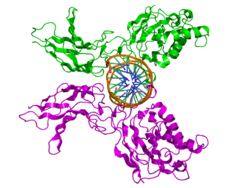
The RHD is composed of two immunoglobulin-like beta barrel subdomains that grip the DNA in the major groove. The N-terminal specificity domain resembles the core domain of the p53 transcription factor, and contains a recognition loop that interacts with DNA bases. In the case of NF-κB, the C-terminal dimerization subdomain determines dimerization propensity with other proteins in the NF-κB/Rel protein family. The dimerization subdomain is immediately followed by a nuclear localization sequence that also comprises the site for inhibitory interactions with IκB.[4]
References
- ↑ "NF-κB Rel subunit exchange on a physiological timescale". Protein Science 30 (9): 1818–1832. September 2021. doi:10.1002/pro.4134. PMID 34089216.
- ↑ "Combinatorial transcription factors". Current Opinion in Genetics & Development 8 (5): 552–559. October 1998. doi:10.1016/S0959-437X(98)80010-5. PMID 9794820.
- ↑ "cis-acting, element-specific transcriptional activity of differentially phosphorylated nuclear factor-kappa B". The Journal of Biological Chemistry 280 (1): 244–252. January 2005. doi:10.1074/jbc.M409344200. PMID 15516339.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid7830764
 |

