Biology:TOX
 Generic protein structure example |
Thymocyte selection-associated high mobility group box protein TOX is a protein that in humans is encoded by the TOX gene.[1][2][3] TOX drives T-cell exhaustion[4][5] and plays a role in innate lymphoid cell development.[6][7]
Structure
The TOX gene encodes a protein that belongs to a large superfamily of chromatin associated proteins that share an approximately 75 amino acid DNA binding motif, the HMG (high mobility group)-box (named after that found in the canonical member of the family, high mobility group protein 1). Some high mobility group (HMG) box proteins (e.g., LEF1) contain a single HMG box motif and bind DNA in a sequence-specific manner, while other members of this family (e.g., HMGB1) have multiple HMG boxes and bind DNA in a sequence-independent but structure-dependent manner. While TOX has a single HMG-box motif,[3] it is predicted to bind DNA in a sequence-independent manner.[8]
TOX subfamily
TOX is a member of a small subfamily of proteins (TOX2, TOX3, and TOX4) that share almost identical HMG-box sequences.[8] TOX2 has been identified to play a role in the differentiation of T follicular helper cell.[9] TOX2 is thought to be a downstream signal of BCL-6.[9] TOX3 has been identified as a breast cancer susceptibility locus.[10][11] TOX is highly expressed in the thymus, the site of development of T lymphocytes.[6] Knockout mice that lack TOX have a severe defect in development of certain subsets of T lymphocytes.[12]
Function
T cell exhaustion
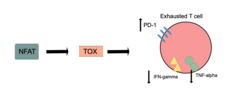
TOX is necessary for T cell persistence but also drives T cell exhaustion.[13][14][15] An increase in TOX expression is characterized by a weakening of the effector functions of the cytotoxic T cell and upregulation of inhibitory receptors on the cytotoxic T cells.[16][17] TOX promotes the exhausted T cell phenotype through epigenetic remodeling.[16][18] PD-1 is an inhibitory marker on T cells that increases when TOX is unregulated.[16][19][18] This allows for cancerous cells to evade the cytotoxic T cells through upregulated expression of PD-L1.[20]
Effector function
Markers of effector functions that are decreased when TOX is overexpressed are KLRG1, TNF, and IFN-gamma.[4] IFN-gamma and TNF-alpha production are also increased when the Tox and Tox2 genes are deleted.[5] Upregulation of effector function in cells lacking TOX is not always seen and it has been proposed that inhibitory receptor function is separated from effector CD8+ cytotoxic T cell function.[4] T-cell exhaustion does not occur when TOX is deleted from CD8+ T cells, but the cells instead adopt the KLRG1+ terminal effector state and undergo apoptosis, or programmed cell death.[5] It was therefore proposed that TOX prevents this terminal differentiation and instead promotes exhaustion so that the T-cell has a slightly more sustained response.[5]
Cancer & chronic infection
In cancer or during chronic viral infection, T-cell exhaustion occurs when cytotoxic T-cells are constantly stimulated.[4][21] TOX is upregulated in CD8+ T cells from chronic infection when compared to acute infection.[4] Patients with cancer typically have high levels of TOX in their tumor-infiltrating lymphocytes,[4] and anti-tumor immunity is heightened when Tox and Tox2 are deleted.[5] TOX and TOX2-deficient tumor-specific CAR T cells additionally have increased antitumor effector cell function as well as decreased levels of inhibitory receptors.[4]
Activation
NFAT transcription factors are essential for activating TOX in CD8+ T-cells,[4] and it has been suggested that TOX is a downstream target of NFAT.[5] The expression and function of NR4a (a target of NFAT) and TOX are strongly linked with reduced NR4a expression in Tox double knockout T cells and minimized Tox expression in NR4a triple knockout T cells.[5]
T-cell development
TOX is necessary for positive selection in developing thymocytes.[22] Knock out TOX mice shows a requirement of TOX for the CD4 T cell lineage,[22] however CD8 single positive T-cells were still able to develop.[22]
Innate lymphoid cells development
TOX is necessary for the development of innate lymphoid cells.[6][7] Innate lymphoid cells include ILC1, ILC2, ILC3 and NK cells.[22]
Notch signaling can aid in the development of all innate lymphoid cells, but in TOX-deficient cells, Notch target genes are expressed at low levels, so it is possible that TOX is required for downstream activation of these Notch target genes.[6] TOX was also found to bind Hes1, a Notch target gene, in embryonic kidney cells.[6]
Several ILC3 populations are reduced in the absence of TOX, implicating TOX’s role in their development.[6] In the small intestine, major ILC3 populations are normal in TOX-deficient cells, suggesting that gut ILC3 development may occur independently of TOX.[6] Some ILC3 populations in the gut expand in the absence of TOX.[6]
It has been proposed that NFIL3 and TOX regulate the transition of common lymphoid progenitor to early innate lymphoid progenitor.[7] In NFIL3-deficient mice, the expression of TOX is downregulated, indicating that NFIL3 is directly affecting the expression of TOX which is then acting downstream in ILC development.[7] TOX-deficient mice and NFIL3-deficient mice both lack mature ILCs and ILC progenitors.[7]
References
- ↑ "Prediction of the coding sequences of unidentified human genes. XI. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro". DNA Research 5 (5): 277–286. October 1998. doi:10.1093/dnares/5.5.277. PMID 9872452.
- ↑ "TOX: an HMG box protein implicated in the regulation of thymocyte selection". Nature Immunology 3 (3): 272–280. March 2002. doi:10.1038/ni767. PMID 11850626.
- ↑ 3.0 3.1 "Entrez Gene: thymocyte selection-associated high mobility group box gene TOX". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=9760.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 "TOX for tired T cells". Nature Reviews. Immunology 19 (8): 476. August 2019. doi:10.1038/s41577-019-0193-9. PMID 31243349.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 "Memory T cell, exhaustion, and tumor immunity". Immunological Medicine 43 (1): 1–9. March 2020. doi:10.1080/25785826.2019.1698261. PMID 31822213.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 "The Role of TOX in the Development of Innate Lymphoid Cells". Mediators of Inflammation 2015: 243868. 2015. doi:10.1155/2015/243868. PMID 26556952.
- ↑ 7.0 7.1 7.2 7.3 7.4 "NK Cell Development in Times of Innate Lymphoid Cell Diversity" (in English). Frontiers in Immunology 11: 813. 2020. doi:10.3389/fimmu.2020.00813. PMID 32733432.
- ↑ 8.0 8.1 "TOX defines a conserved subfamily of HMG-box proteins". BMC Genomics 4 (1): 13. April 2003. doi:10.1186/1471-2164-4-13. PMID 12697058.
- ↑ 9.0 9.1 "TOX2 helping hand for TFH cells". Nature Reviews. Immunology 20 (1): 4–5. January 2020. doi:10.1038/s41577-019-0249-x. PMID 31745259.
- ↑ "Genome-wide association study identifies novel breast cancer susceptibility loci". Nature 447 (7148): 1087–1093. June 2007. doi:10.1038/nature05887. PMID 17529967. Bibcode: 2007Natur.447.1087E.
- ↑ "Common variants on chromosomes 2q35 and 16q12 confer susceptibility to estrogen receptor-positive breast cancer". Nature Genetics 39 (7): 865–869. July 2007. doi:10.1038/ng2064. PMID 17529974.
- ↑ "Development of all CD4 T lineages requires nuclear factor TOX". The Journal of Experimental Medicine 205 (1): 245–256. January 2008. doi:10.1084/jem.20071944. PMID 18195075.
- ↑ "TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection". Nature 571 (7764): 265–269. July 2019. doi:10.1038/s41586-019-1326-9. PMID 31207605. https://mediatum.ub.tum.de/1538141.
- ↑ "TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion". Nature 571 (7764): 211–218. July 2019. doi:10.1038/s41586-019-1325-x. PMID 31207603.
- ↑ "TOX is a critical regulator of tumour-specific T cell differentiation". Nature 571 (7764): 270–274. July 2019. doi:10.1038/s41586-019-1324-y. PMID 31207604.
- ↑ 16.0 16.1 16.2 "TOX for tired T cells". Nature Reviews. Immunology 19 (8): 476. August 2019. doi:10.1038/s41577-019-0193-9. PMID 31243349.
- ↑ "Defining 'T cell exhaustion'". Nature Reviews. Immunology 19 (11): 665–674. November 2019. doi:10.1038/s41577-019-0221-9. PMID 31570879.
- ↑ 18.0 18.1 "Role, function and regulation of the thymocyte selection-associated high mobility group box protein in CD8+ T cell exhaustion". Immunology Letters 229: 1–7. January 2021. doi:10.1016/j.imlet.2020.11.004. PMID 33186634.
- ↑ "Single-cell transcriptome analysis reveals TOX as a promoting factor for T cell exhaustion and a predictor for anti-PD-1 responses in human cancer". Genome Medicine 12 (1): 22. February 2020. doi:10.1186/s13073-020-00722-9. PMID 32111241.
- ↑ "CD8+ T cell states in human cancer: insights from single-cell analysis". Nature Reviews. Cancer 20 (4): 218–232. April 2020. doi:10.1038/s41568-019-0235-4. PMID 32024970.
- ↑ "CD8+ T cell differentiation and dysfunction in cancer". Nature Reviews. Immunology 22 (4): 209–223. July 2021. doi:10.1038/s41577-021-00574-3. PMID 34253904.
- ↑ 22.0 22.1 22.2 22.3 "The many roles of TOX in the immune system". Current Opinion in Immunology 24 (2): 173–177. April 2012. doi:10.1016/j.coi.2011.12.001. PMID 22209117.
Further reading
- "Construction of expression-ready cDNA clones for KIAA genes: manual curation of 330 KIAA cDNA clones". DNA Research 9 (3): 99–106. June 2002. doi:10.1093/dnares/9.3.99. PMID 12168954.
- "Fetal hemoglobin in sickle cell anemia: Bayesian modeling of genetic associations". American Journal of Hematology 83 (3): 189–195. March 2008. doi:10.1002/ajh.21048. PMID 17918249.
- "TOX provides a link between calcineurin activation and CD8 lineage commitment". The Journal of Experimental Medicine 199 (8): 1089–1099. April 2004. doi:10.1084/jem.20040051. PMID 15078895.
 |

