Biology:Anti-sigma factors
Introduction
Anti-sigma factors are small proteins that bind to sigma factors and inhibit transcriptional activity in regulating prokaryote gene expression. Anti-sigma factors have both a sigma-binding domain and a sensory/signaling domain; this allows them to respond to signals inside and outside the cell.[1] Anti-sigma factors have been found in several bacteria, including Escherichia coli and Salmonella, and viruses such as the T4 bacteriophage. Anti-sigma factors have an antagonistic effect on sigma factors.[2] Each sigma factor has an associated anti-sigma factor that regulates it. These anti-sigma factors are divided into cytoplasmic-bound anti-sigma factors and inner membrane-bound anti-sigma factors. The differences in these sigma factors are where in the cell they are bound. Cytoplasmic-bound anti-sigma factors include FlgM, DnaK, RssB, and HscC. Inner membrane-bound anti-sigma factors, also called extra-cytoplasmic function (ECF) anti-sigma factors, include FecR and RseA. ECF anti-sigma factors tend to be more diverse in genetic sequence than cytoplasmic-bound anti-sigma factors.[3] These factors regulate many cellular processes, such as flagellum assembly, transport of materials, cell growth, and the cell's stress response.[4]
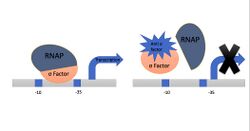
Sigma factors are essential proteins that start the transcription by binding with RNAP; anti-sigma factors are proteins that inhibit the activities of sigma factors affected by several mechanisms. These mechanisms include adding up the anti-sigma factor between sigma or twisting the anti-sigma factor around sigma—gene regulation, especially in bacteria, allows for adaptivity and normal cell differentiation and development. Gene regulation has many different layers of regulators. Yet, initiating transcription is crucial in controlling which genes are expressed.[5]
Anti-sigma factors are simultaneously transcribed with their associated sigma factor. This pairing creates a negative feedback loop, maintaining proper levels of both contrasting factors as there can only be one anti-sigma factor per sigma factor that is transcribed.[5]
Research shows anti-sigma factors have more activities than contouring sigma factors effects. Anti-sigma factors can also activate some cells while inhibiting others, meaning they have an essential role in cell function.[5][6]
Mechanism
There are three main categories for triggering the release of sigmas factors from anti-sigma factors: partner switching, direct signaling, and a mechanism regulated by proteolysis.[1]
The partner-switching mechanism is commonly found in Gram-positive bacteria. It consists of four key players: a sigma factor, an anti-sigma factor, an anti-anti-sigma factor, and an input phosphatase complex. A cell that is not under stress has an anti-sigma factor that is bound to the sigma factor on the gene and keeps it inactive. In times of stress, a phosphatase complex dephosphorylates the anti-sigma factor, allowing the anti-sigma factor to switch partners and bind to the anti-anti-sigma factor. This frees the sigma factors to activate the gene. Environmental stressors, such as heat, often activate this mechanism.[1]
The direct signaling mechanism is as it sounds: the anti-sigma factor binds to a signal, which causes conformation changes in the structure of the anti-sigma factors, resulting in the release of the sigma factors.[1]
The regulated intramembrane proteolysis (RIP) mechanism allows signal transduction across membranes. This mechanism is often used to regulate ECF sigma factors. The mechanism involves two sequential cleavages, the first being an external cleavage of membrane-traversing anti-sigma factor and the second cleavage of the anti-sigma factors in the membrane's plane, resulting in a free cytoplasmic domain.[1]
Anti-sigma factors in Escherichia coli
E. coli has seven main sigma factors, five of which have a specific anti-sigma factor. The anti-sigma factor binding to its sigma factors depends upon environmental cues. This mechanism blocks the transcription of genes that are unnecessary in new conditions. The table below shows five sigma factors, what process it affects, and its corresponding anti-sigma factor. In E. coli, sigma factors transcribe their anti-sigma factors; this creates a negative feedback loop. The sigma factor can be regulated when the anti-sigma factor is transcribed and the anti-sigma factor when the sigmas gene is transcribed. Sigma factors 70 and 54 don't have specific anti-sigma factors; they have other negative feedback loop mechanisms.[4]
| Sigma factor | Sigma Effect | Related anti-sigma factor |
| σ38 | Master regulator of general stress response | RssB |
| σ32 | Heat shock response ≥ 37 °C | Dnak |
| σ28 | Active late gene of flagellum assembly | FIgM |
| σ24 | Signals release of factors to fix misfolded proteins | RseA |
| σ19 | One signal in the EC signaling pathway | FecR |
Anti-Anti-Sigma Factors
Anti-anti-sigma factors allow for the dissociation of the matching anti-sigma factor from its sigma factors, thought binding to the anti-sigma factor, forcing its release from the sigma factor. This allows for tighter regulation of the transcription of genes as a response to environmental conditions. Anti-anti-sigma factors can thereby function as negative or positive regulatory elements, depending on the corroding sigma factor and gene involved.[7][8]
In Bacteriophage
T4 bacteriophage uses anti-sigma factor to ruin the Escherichia coli polymerase in order that direct exclusive transcription of its own genes.
AsiA is an anti-sigma factor gene that is required for bacteriophage T4 to be developed). Which means that AsiA is an essential anti-sigma factor in bacteriophage.[6][4][9][8]
Sigma B Factor in Bacillus subtilis
Sigma B was the first anti-sigma factor identified in a bacterium. It is found in Bacillus subtilis and other similar bacteria. Sigma B is a stress response factor that plays a role in survival and against destruction that could be caused by other organisms such as mammals. General stress responses that are controlled by Sigma B are stimulated by things like temperature, salt concentration, energy depletion, etc. Once activated, Sigma B binds to the RNAP and recognizes a promoter, causing inhibition of the stimuli. Because Sigma B orthologs are conserved in various gram-positive bacteria, this anti-sigma factor plays an essential role in the evolution of different bacteria and their ability to respond to stressing factors. Scientist have found that the anti- sigma factor, Sigma B controls more than 150 genes that are influential in stress response.[10][11]
RsbW in Bacillus subtilis
When Bacillus subtilis is not under stress conditions, it is negatively regulated by the anti-sigma factor, Rsbw. RsbW is an anti-sigma factor that regulates another anti-sigma factor , sigma B. RsbW binds to sigma B and prevents it from forming an RNA polymerase holoenzyme. However, in stressed conditions, the unphosphorylated form of the protein, RsbV, competes with Sigma B for binding to RsbW. RsbV binds to RsbW, allowing sigma B to bind to the core RNA polymerase, resulting in the expression of stress response.[12][13]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Bacterial Sigma Factors and Anti-Sigma Factors: Structure, Function and Distribution". Biomolecules 5 (3): 1245–65. June 2015. doi:10.3390/biom5031245. PMID 26131973.
- ↑ Hofmann, Nina; Wurm, Reinhild; Wagner, Rolf (2011-05-06). "The E. coli Anti-Sigma Factor Rsd: Studies on the Specificity and Regulation of Its Expression". PLOS ONE 6 (5): e19235. doi:10.1371/journal.pone.0019235. ISSN 1932-6203. PMID 21573101. Bibcode: 2011PLoSO...619235H.
- ↑ Helmann, John D. (2002). The extracytoplasmic function (ECF) sigma factors. Advances in Microbial Physiology. 46. pp. 47–110. doi:10.1016/s0065-2911(02)46002-x. ISBN 9780120277469. https://pubmed.ncbi.nlm.nih.gov/12073657/.
- ↑ 4.0 4.1 4.2 "Anti-Sigma Factors in E. coli: Common Regulatory Mechanisms Controlling Sigma Factors Availability". Current Genomics 14 (6): 378–87. September 2013. doi:10.2174/1389202911314060007. PMID 24396271.
- ↑ 5.0 5.1 5.2 Hughes, Kelly T.; Mathee, Kalai (October 1998). "The Anti-Sigma Factors" (in en). Annual Review of Microbiology 52 (1): 231–286. doi:10.1146/annurev.micro.52.1.231. ISSN 0066-4227. PMID 9891799. https://www.annualreviews.org/doi/10.1146/annurev.micro.52.1.231.
- ↑ 6.0 6.1 "RsrA, an anti-sigma factor regulated by redox change". The EMBO Journal 18 (15): 4292–8. August 1999. doi:10.1093/emboj/18.15.4292. PMID 10428967.
- ↑ (in en) Molecular Biology. ISBN 9780123785947. https://www.sciencedirect.com/book/9780123785947/molecular-biology. Retrieved 2023-12-02.
- ↑ 8.0 8.1 Sevcikova, Beatrica; Rezuchova, Bronislava; Homerova, Dagmar; Kormanec, Jan (November 2010). "The Anti-Anti-Sigma Factor BldG Is Involved in Activation of the Stress Response Sigma Factor σH in Streptomyces coelicolor A3(2)". Journal of Bacteriology 192 (21): 5674–5681. doi:10.1128/JB.00828-10. PMID 20817765.
- ↑ "Mutational analysis of RsrA, a zinc-binding anti-sigma factor with a thiol-disulphide redox switch". Molecular Microbiology 39 (4): 1036–47. February 2001. doi:10.1046/j.1365-2958.2001.02298.x. PMID 11251822.
- ↑ Kazmierczak, Mark J.; Wiedmann, Martin; Boor, Kathryn J. (December 2005). "Alternative Sigma Factors and Their Roles in Bacterial Virulence". Microbiology and Molecular Biology Reviews 69 (4): 527–543. doi:10.1128/MMBR.69.4.527-543.2005. ISSN 1092-2172. PMID 16339734.
- ↑ Rodriguez Ayala, Facundo; Bartolini, Marco; Grau, Roberto (2020-09-15). "The Stress-Responsive Alternative Sigma Factor SigB of Bacillus subtilis and Its Relatives: An Old Friend With New Functions". Frontiers in Microbiology 11: 1761. doi:10.3389/fmicb.2020.01761. ISSN 1664-302X. PMID 33042030.
- ↑ Benson, A. K.; Haldenwang, W. G. (1993-03-15). "Bacillus subtilis sigma B is regulated by a binding protein (RsbW) that blocks its association with core RNA polymerase". Proceedings of the National Academy of Sciences of the United States of America 90 (6): 2330–2334. doi:10.1073/pnas.90.6.2330. ISSN 0027-8424. PMID 8460143. Bibcode: 1993PNAS...90.2330B.
- ↑ Rodriguez Ayala, Facundo; Bartolini, Marco; Grau, Roberto (2020). "The Stress-Responsive Alternative Sigma Factor SigB of Bacillus subtilis and Its Relatives: An Old Friend With New Functions". Frontiers in Microbiology 11: 1761. doi:10.3389/fmicb.2020.01761. ISSN 1664-302X. PMID 33042030.
Further reading
- "RsrA, an anti-sigma factor regulated by redox change". The EMBO Journal 18 (15): 4292–8. August 1999. doi:10.1093/emboj/18.15.4292. PMID 10428967.
- "A novel transcriptional regulation mechanism in the flagellar regulon of Salmonella typhimurium: an antisigma factor inhibits the activity of the flagellum-specific sigma factor, sigma F". Molecular Microbiology 6 (21): 3149–57. November 1992. doi:10.1111/j.1365-2958.1992.tb01771.x. PMID 1453955.
- "Anti-sigma factors". Current Opinion in Microbiology 2 (2): 135–41. April 1999. doi:10.1016/S1369-5274(99)80024-1. PMID 10322161.
- "The anti-anti-sigma factor BldG is involved in activation of the stress response sigma factor σ(H) in Streptomyces coelicolor A3(2)". Journal of Bacteriology 192 (21): 5674–81. November 2010. doi:10.1128/JB.00828-10. PMID 20817765.
 |

