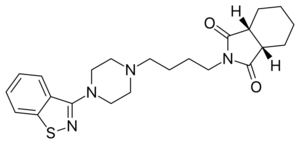
Chemistry:Perospirone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Lullan |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 92%[1] |
| Metabolism | Hepatic[1] |
| Elimination half-life | 1.9–2.5 hours[1][2] |
| Excretion | Renal (0.4% as unchanged drug)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C23H30N4O2S |
| Molar mass | 426.58 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Perospirone (Lullan) is an atypical antipsychotic of the azapirone family.[1] It was introduced in Japan by Dainippon Sumitomo Pharma in 2001 for the treatment of schizophrenia and acute cases of bipolar mania.[3][4]
Medical uses
Its primary uses are in the treatment of schizophrenia and bipolar mania.[3][4]
Schizophrenia
In a clinical trial that compared it to haloperidol in the treatment of schizophrenia it was found to produce significantly superior overall symptom control.[5] In another clinical trial perospirone was compared with mosapramine and produced a similar reduction in total PANSS score, except with respect to the blunted affect part of the PANSS negative score, in which perospirone produced a significantly greater improvement.[6] In an open-label clinical trial comparing aripiprazole with perospirone there was no significant difference between the two treatments discovered in terms of both efficacy and tolerability.[7] In 2009 a clinical trial found that perospirone produced a similar reduction of PANSS score than risperidone and the extrapyramidal side effects was similar in both frequency and severity between groups.[8]
A meta-analysis published in 2013 found that it is statistically significantly less efficacious than other second-generation antipsychotics.[9]
Adverse effects
Has a higher incidence of extrapyramidal side effects than the other atypical antipsychotics, but still less than that seen with typical antipsychotics.[1][10] A trend was observed in a clinical trial comparing mosapramine with perospirone that favoured perospirone for producing less prominent extrapyramidal side effects than mosapramine although statistical significant was not reached.[6] It may produce less QT interval prolongation than zotepine, as in one patient who had previously been on zotepine switching to perospirone corrected their prolonged QT interval.[11] It also tended to produce less severe extrapyramidal side effects than haloperidol in a clinical trial comparing the two (although statistical significance was not reached).[5]
Discontinuation
The British National Formulary recommends a gradual withdrawal when discontinuing antipsychotics to avoid acute withdrawal syndrome or rapid relapse.[12] Symptoms of withdrawal commonly include nausea, vomiting, and loss of appetite.[13] Other symptoms may include restlessness, increased sweating, and trouble sleeping.[13] Less commonly there may be a felling of the world spinning, numbness, or muscle pains.[13] Symptoms generally resolve after a short period of time.[13]
There is tentative evidence that discontinuation of antipsychotics can result in psychosis.[14] It may also result in reoccurrence of the condition that is being treated.[15] Rarely tardive dyskinesia can occur when the medication is stopped.[13]
Pharmacology
Perospirone binds to the following receptors with very high affinity (as an antagonist unless otherwise specified):[9][16][17][18][19][20]
And the following receptor with high affinity:[9]
- H1 (inverse agonist)
And the following with moderate affinity:[9]
- D4
- α1 adrenoceptor
And with low affinity for the following receptor:[9]
- D1
See also
- Azapirone
- Blonanserin — another second-generation antipsychotic that's only approved for clinical use in East Asia
- Mosapramine
- Zotepine
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "Perospirone". CNS Drugs 15 (4): 329–37; discussion 338. 2001. doi:10.2165/00023210-200115040-00006. PMID 11463136.
- ↑ "Steady-state pharmacokinetics of a new antipsychotic agent perospirone and its active metabolite, and its relationship with prolactin response". Therapeutic Drug Monitoring 26 (4): 361–365. August 2004. doi:10.1097/00007691-200408000-00004. PMID 15257064.
- ↑ 3.0 3.1 "Perospirone (Sumitomo Pharmaceuticals)". Current Opinion in Investigational Drugs 3 (1): 121–129. January 2002. PMID 12054062.
- ↑ 4.0 4.1 "Now on the Market : New Antipsychotic "Lullan® Tablets" - serotonin-dopamine antagonist originated in Japan". Sumitomo Pharmaceuticals 2001 | News Release | Dainippon Sumitomo Pharma. 8 February 2001. http://www.ds-pharma.co.jp/english/news/sumitomo_2001/no_002.html.
- ↑ 5.0 5.1 "Clinical evaluation of a new antipsychotic, perospirone HCl, on schizophrenia: a comparative double-blind study with haloperidol". Rinsho Hyoka 24 (2–3): 159–205. 1997.
- ↑ 6.0 6.1 "Clinical evaluation of a serotonin-2 and dopamine-2 receptor antagonist (SDA), perospirone HCl on schizophrenia: a comparative double-blind study with mosapramine HCl". Rinsho Hyoka 24 (2–3): 207–48. 1997.
- ↑ "A 12-week randomized, open-label study of perospirone versus aripiprazole in the treatment of Japanese schizophrenia patients". Progress in Neuro-Psychopharmacology & Biological Psychiatry 40: 110–114. January 2013. doi:10.1016/j.pnpbp.2012.09.010. PMID 23022672.
- ↑ "Randomized clinical comparison of perospirone and risperidone in patients with schizophrenia: Kansai Psychiatric Multicenter Study". Psychiatry and Clinical Neurosciences 63 (3): 322–328. June 2009. doi:10.1111/j.1440-1819.2009.01947.x. PMID 19566763.
- ↑ 9.0 9.1 9.2 9.3 9.4 "Efficacy and tolerability of perospirone in schizophrenia: a systematic review and meta-analysis of randomized controlled trials". CNS Drugs 27 (9): 731–741. September 2013. doi:10.1007/s40263-013-0085-7. PMID 23812802.
- ↑ "Perospirone Hydrochloride". Martindale: The Complete Drug Reference. The Royal Pharmaceutical Society of Great Britain. 23 September 2011. http://www.medicinescomplete.com/mc/martindale/current/15232-k.htm. Retrieved 3 November 2013.
- ↑ "Improvement in QTc prolongation induced by zotepine following a switch to perospirone". Psychiatry and Clinical Neurosciences 66 (3): 244. April 2012. doi:10.1111/j.1440-1819.2012.02321.x. PMID 22443250.
- ↑ Joint Formulary Committee, BMJ, ed (March 2009). "4.2.1". British National Formulary (57 ed.). United Kingdom: Royal Pharmaceutical Society of Great Britain. p. 192. ISBN 978-0-85369-845-6. "Withdrawal of antipsychotic drugs after long-term therapy should always be gradual and closely monitored to avoid the risk of acute withdrawal syndromes or rapid relapse."
- ↑ 13.0 13.1 13.2 13.3 13.4 (in en) Adverse Syndromes and Psychiatric Drugs: A Clinical Guide. OUP Oxford. 2004. p. 207-216. ISBN 9780198527480. https://books.google.com/books?id=CWR7DwAAQBAJ&pg=PA207.
- ↑ "Does antipsychotic withdrawal provoke psychosis? Review of the literature on rapid onset psychosis (supersensitivity psychosis) and withdrawal-related relapse". Acta Psychiatrica Scandinavica 114 (1): 3–13. July 2006. doi:10.1111/j.1600-0447.2006.00787.x. PMID 16774655.
- ↑ (in en) Adherence to Antipsychotics in Schizophrenia. Springer Science & Business Media. 2013. p. 85. ISBN 9788847026797. https://books.google.com/books?id=odE-AgAAQBAJ&pg=PA85.
- ↑ "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. 12 January 2011. http://pdsp.med.unc.edu/pdsp.php.
- ↑ "Pharmacological actions of SM-9018, a new neuroleptic drug with both potent 5-hydroxytryptamine2 and dopamine2 antagonistic actions". Japanese Journal of Pharmacology 53 (3): 321–329. July 1990. doi:10.1254/jjp.53.321. PMID 1975278.
- ↑ "Binding profile of SM-9018, a novel antipsychotic candidate". Japanese Journal of Pharmacology 54 (4): 478–481. December 1990. doi:10.1254/jjp.54.478. PMID 1982326.
- ↑ "5-HT1A receptor agonist properties of antipsychotics determined by [35S]GTPgammaS binding in rat hippocampal membranes". Clinical and Experimental Pharmacology & Physiology 34 (5–6): 462–466. 2007. doi:10.1111/j.1440-1681.2007.04595.x. PMID 17439416.
- ↑ "Antipsychotic drugs which elicit little or no parkinsonism bind more loosely than dopamine to brain D2 receptors, yet occupy high levels of these receptors". Molecular Psychiatry 3 (2): 123–134. March 1998. doi:10.1038/sj.mp.4000336. PMID 9577836.
 |

