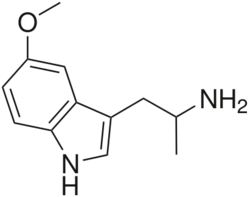
Chemistry:5-MeO-aMT
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C12H16N2O |
| Molar mass | 204.273 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 216 to 218 °C (421 to 424 °F) |
| |
| |
| | |
5-MeO-aMT or 5-methoxy-α-methyltryptamine, α,O-Dimethylserotonin (Alpha-O) is a potent psychedelic tryptamine. It is soluble in ethanol.
Pharmacology
The sympathomimetic effects may in turn be caused by 5-MeO-AMT's structural similarity to the amphetamines. As noted by Alexander Shulgin, the alpha-methylated tryptamines can be looked at as the tryptamine homologues of the amphetamines (alpha-methylated phenethylamines).
Mechanisms of action such as inhibition of monoamine reuptake may be involved also.[2]
Pharmacokinetics
| Binding Sites | Binding Affinity Ki (μM)[3] |
|---|---|
| 5-HT1A | 0.046 |
| 5-HT2A | 0.034 |
| 5-HT2C | 0.09 |
| D1 | >25 |
| D2 | >25 |
| D3 | >25 |
| α1A | >12 |
| α2A | 11 |
| TAAR1 | 1.1 |
| H1 | >25 |
| SERT | 12 |
| DAT | >26 |
| NET | >22 |
Recreational usage
5-MeO-AMT is supposedly sold in 4 mg tablets by the street name Alpha-O and taken as a recreational drug. Since the DEA arrests of the makers of a huge percentage of the United States' LSD in 2000, 5-MeO-AMT may have occasionally been sold under the guise of LSD in liquid, sugar cube, or blotter form, though this may be due to DEA reports of finding it on sugar cubes and blotters like LSD.[4][5]
The most common route of administration for 5-MeO-AMT is orally. Anecdotal reports, however, have described snorting or smoking the substance. Intravenous (IV) and intramuscular (IM) routes are rarely, if ever, used outside research settings due to the high potency, powerful effects and quicker onset.
Effects
The effects of 5-MeO-AMT occur at 4–7 mg orally for most users.
Erowid lists the following effects:[6]
Positive
- Increased energy
- Improved mood heading into euphoria at higher doses
- Increased sociability, gregariousness
- Increased giggling and laughter
- Increased sense of creative thinking
- Increased pleasure from sense of touch
- Intensification of sex for some users
Neutral
- Lightheadedness
- Brightening of colors
- Visuals including motion, waves, breathing walls, etc. (usually at higher doses, over 4–5 mg)
- Increased attention to detail
- Auditory distortions and/or hallucinations (usually at higher doses)
Negative
- Headache
- Body fatigue
- Chills from elevated body temperature (potential dehydration)
- Stress and extreme fatigue from long duration of effects.
- Nausea, diarrhea
- Vomiting
- Difficulty sleeping or resting for 12–24 hours after ingestion.
- Paranoia, irritability, anxiety (increasing with dose).
- Delusional, aggressive, or dissociated behaviour at very high doses (20+ mg)
- Risk of death
Dangers
If misrepresented as LSD, 5-MeO-AMT can be extremely dangerous; users may take a number of "hits" of 5-MeO-AMT, assuming that it is LSD. Unlike LSD, which is very safe in overdose, 5-MeO-AMT can be very harmful or fatal. Particularly sensitive individuals can experience symptoms of overdose at dosages in the normal (for most users) range — as low as 20 mg. This has led to at least a few hospitalizations and possibly more than one death.[7][8] It is likely that the overdose potential of the compound is due to its sympathomimetic effects, as the side effects noted in overdose cases include cardiac arrhythmia and seizure. It also seems that oral consumption is safer than insufflation.[citation needed]
Gloria Discerni, 18, died after overdosing on a drug initially believed to be LSD. Authorities learned months later that the drug wasn't LSD but a "designer drug" identified as 5-MeO-AMT.[9]
Legal Status
Australia
5-MeO-aMT is considered a Schedule 9 prohibited substance in Australia under the Poisons Standard (October 2015).[10] A Schedule 9 substance is a substance which may be abused or misused, the manufacture, possession, sale or use of which should be prohibited by law except when required for medical or scientific research, or for analytical, teaching or training purposes with approval of Commonwealth and/or State or Territory Health Authorities.[10]
Sweden
Sveriges riksdags health ministry Statens folkhälsoinstitut classified 5-MeO-αMT as "health hazard" under the act Lagen om förbud mot vissa hälsofarliga varor (translated Act on the Prohibition of Certain Goods Dangerous to Health) as of Oct 1, 2004, in their regulation SFS 2004:696 listed as 5-metoxi-alfametyltryptamin (5-MeO-AMT), making it illegal to sell or possess.[11]
United Kingdom
5-MeO-αMT was made illegal in the United Kingdom as of 7 January 2015, along with 5-MeO-DALT. This was following the events of 10 June 2014 when the Advisory Council on the Misuse of Drugs recommended that 5-MeO-αMT be scheduled as a class A drug by updating the blanket ban clause on tryptamines.
United States
5-MeO-AMT is unscheduled at the federal level in the United States .[12] However, the DEA considers the chemical a controlled substance analogue.[13] The agency’s opinion on this matter may change at any time.
Florida
5-MeO-AMT is a Schedule I controlled substance in the state of Florida, making it illegal to buy, sell, or possess.[14]
See also
- 5-Chloro-αMT
- 5-EtO-AMT
- 5-MeO-AET
- 5-MeO-DMT
- 5-MeO-DPT
- 5-MeO-DiPT
- AMT
References
- ↑ "5-MeO-AMT; Fast Facts". January 1, 2006. https://www.justice.gov/archive/ndic/pubs9/9576/index.htm.
- ↑ "The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain". European Journal of Pharmacology 559 (2–3): 132–137. March 2007. doi:10.1016/j.ejphar.2006.11.075. PMID 17223101.
- ↑ "Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens". European Neuropsychopharmacology 26 (8): 1327–1337. August 2016. doi:10.1016/j.euroneuro.2016.05.001. PMID 27216487. http://edoc.unibas.ch/53326/1/20170117174852_587e4af45b658.pdf.
- ↑ "The Identification of 5-Methoxy-alpha-methyltryptamine (5-MeO-AMT)". Microgram Journal (Drug Enforcement Administration) 1. January–June 2003. https://www.justice.gov/dea/programs/forensicsci/microgram/journal071203/mj071203_pg1.html. Retrieved 2011-09-16.
- ↑ "Police Reports of 5-MeO-AMT". Erowid Vault. http://www.erowid.org/chemicals/5meo_amt/5meo_amt_info2.shtml.
- ↑ "5-MeO-AMT Vault: Effects". Erowid Vault. http://www.erowid.org/chemicals/5meo_amt/5meo_amt_effects.shtml.
- ↑ "Reported LSD-Related death was not LSD". Erowid Vault. July 2007. http://www.erowid.org/chemicals/lsd/lsd_media2.shtml.
- ↑ "5-MeO-AMT Hospitalizations & Possible Deaths". Erowid Vault. January 2007. http://www.erowid.org/chemicals/5meo_amt/5meo_amt_info1.shtml.
- ↑ "Charges dropped in drug death". The Spokesman-Review. 13 December 2006. http://www.spokesman.com/stories/2006/dec/13/charges-dropped-in-drug-death/.
- ↑ 10.0 10.1 "Poisons Standard". Federal Register of Legislation. Australian Government. October 2015. https://www.comlaw.gov.au/Details/F2015L01534.
- ↑ "Förordning om ändring i förordningen (1999:58) om förbud mot vissa hälsofarliga varor" (in Swedish). Swedish Code of Statutes. 19 August 2004. http://www.notisum.se/rnp/sls/sfs/20040696.pdf.
- ↑ "§1308.11 Schedule I.". Diversion Control Division. Drug Enforcement Administration, U.S. Department of Justice. http://www.deadiversion.usdoj.gov/21cfr/cfr/1308/1308_11.htm.
- ↑ "5-MeO-AMT; Fast Facts". January 1, 2006. https://www.justice.gov/archive/ndic/pubs9/9576/index.htm.
- ↑ "Chapter 893 - Drug Abuse Prevention and Control". Florida Statutes. http://leg.state.fl.us/statutes/index.cfm?App_mode=Display_Statute&URL=0800-0899/0893/0893.html.
External links
- DEA forensic journal on 5-MeO-AMT
- Erowid 5-MeO-AMT vault
- Lycaeum 5-MeO-AMT page
- TiHKAL entry
- 5-MeO-AMT (α,O-DMS) entry in TiHKAL • info
 |



