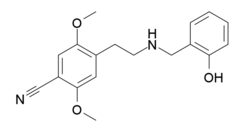
Chemistry:25CN-NBOH
 | |
| Clinical data | |
|---|---|
| Other names | NBOH-2C-CN |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C18H20N2O3 |
| Molar mass | 312.369 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
25CN-NBOH (sometimes also referred to as NBOH-2C-CN)[1] is a compound indirectly derived from the phenethylamine series of hallucinogens, which was discovered in 2014 at the University of Copenhagen.[2] This compound is notable as one of the most selective agonist ligands for the 5-HT2A receptor yet discovered, with a pKi of 8.88 at the human 5-HT2A receptor and with 100x selectivity for 5-HT2A over 5-HT2C, and 46x selectivity for 5-HT2A over 5-HT2B.[3][4][5][6] A tritiated version of 25CN-NBOH has also been accessed and used for more detailed investigations of the binding to 5-HT2 receptors and autoradiography.[7]
Structure
The structure of 25CN-NBOH in complex with an engineered Gαq heterotrimer of the 5-HT2AR has been determined by cryoelectron microscopy (cryo-EM), showing a distinct binding mode when compared to LSD.[8] File:25CN-NBOH.webm
Synthesis
25CN-NBOH is readily available from 2C-H in 57% over 4 steps.[9]
Animal studies
25CN-NBOH was found to partially substitute for DOI but was considerably weaker at inducing a head-twitch response in mice.[10][11] Another in vivo evaluation of 25CN-NBOH concluded that "Given its distinct in vitro selectivity for 5-HT2A over non 5-HT2 receptors and its behavioral dynamics, 25CN-NBOH appears to be a powerful tool for dissection of receptor-specific cortical circuit dynamics, including 5-HT2A related psychoactivity."[12]
25CN-NBOH induces the Head Twitch Response (HTR) also refererred to as "wet dog shakes" in rodents and the cortical fingerprint of serotonin-2A-receptor-mediated shaking behavior has been investigated in detail.[13]
Additional in vivo investigations with this ligand has emerged.[14][15][16][17][18][19][20][21] Chronic administration in mice lead to desensitization of the 5-HT2AR (measured via HTR) and increased startle amplitude[22] whereas it does not effect reversal learning in mice.[23] 25CN-NBOH was shown to increase the production of CTGF in chondrocytes.[24] In rats, 25CN-NBOH induce a reduction in conditioned fear that was countered by pretreatment with 5-HT2AR inverse agonist MDL100907.[25]
A bioanalytical method for the detection of 25CN-NBOH has been developed.[26]
Literature
A review covering the literature up to 2020 was published in 2021.[27]
Related compounds
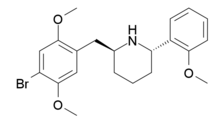
The tendency of the 4-cyano substitution to confer high 5-HT2A selectivity had previously been observed with DOCN,[28] but this was not sufficiently potent to be widely adopted as a research ligand. 25CN-NBOH is still slightly less selective for 5-HT2A than the more complex cyclised derivative 2S,6S-DMBMPP ((2S,6S)-2-(2,5-dimethoxy-4-bromobenzyl)-6-(2-methoxyphenyl)piperidine),[29] in binding assays, however it is also less complex to synthesise and has higher efficacy and selectivity in functional assays as a partial agonist of the 5-HT2A receptor.
Legality
Hungary
25CN-NBOH is illegal in Hungary.[30]
United Kingdom
This substance is a Class A drug in the United Kingdom as a result of the N-benzylphenethylamine catch-all clause in the Misuse of Drugs Act 1971.[31]
See also
References
- ↑ "25CN-NBOH". Chemical Probes. https://www.chemicalprobes.org/25CN-NBOH/.
- ↑ "CNS Medicinal Chemistry in the Kristensen Group". Department of Drug Design and Pharmacology. University of Copenhagen. 25 March 2019. https://drug.ku.dk/disciplines/medicinal-chemistry/kristensen-group/.
- ↑ "Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists". ACS Chemical Neuroscience 5 (3): 243–249. March 2014. doi:10.1021/cn400216u. PMID 24397362.
- ↑ "Detailed Characterization of the In Vitro Pharmacological and Pharmacokinetic Properties of N-(2-Hydroxybenzyl)-2,5-Dimethoxy-4-Cyanophenylethylamine (25CN-NBOH), a Highly Selective and Brain-Penetrant 5-HT2A Receptor Agonist". The Journal of Pharmacology and Experimental Therapeutics 361 (3): 441–453. June 2017. doi:10.1124/jpet.117.239905. PMID 28360333.
- ↑ Hansen M (2011). Design and Synthesis of Selective Serotonin Receptor Agonists for Positron Emission Tomography Imaging of the Brain (Ph.D. thesis). University of Copenhagen.
- ↑ "Effect of 5-HT2A and 5-HT2C receptors on temporal discrimination by mice". Neuropharmacology 107: 364–375. August 2016. doi:10.1016/j.neuropharm.2016.03.038. PMID 27020041.
- ↑ "The selective 5-HT2A receptor agonist 25CN-NBOH: Structure-activity relationship, in vivo pharmacology, and in vitro and ex vivo binding characteristics of [3H]25CN-NBOH". Biochemical Pharmacology 177: 113979. July 2020. doi:10.1016/j.bcp.2020.113979. PMID 32298690.
- ↑ "Structure of a Hallucinogen-Activated Gq-Coupled 5-HT2A Serotonin Receptor" (in English). Cell 182 (6): 1574–1588.e19. September 2020. doi:10.1016/j.cell.2020.08.024. PMID 32946782.
- ↑ "An improved, scalable synthesis of the selective serotonin 2A receptor agonist 25CN-NBOH" (in en). SynOpen 05 (2): a–1524–4439. 2021-06-08. doi:10.1055/a-1524-4439. ISSN 2509-9396.
- ↑ "Hallucinogen-like effects of 2-([2-(4-cyano-2,5-dimethoxyphenyl) ethylaminomethyl)phenol (25CN-NBOH), a novel N-benzylphenethylamine with 100-fold selectivity for 5-HT₂A receptors, in mice"]. Psychopharmacology 232 (6): 1039–1047. March 2015. doi:10.1007/s00213-014-3739-3. PMID 25224567.
- ↑ "Chronic treatment with a metabotropic mGlu2/3 receptor agonist diminishes behavioral response to a phenethylamine hallucinogen". Psychopharmacology 236 (2): 821–830. February 2019. doi:10.1007/s00213-018-5118-y. PMID 30448990.
- ↑ "Tolerance and Tachyphylaxis to Head Twitches Induced by the 5-HT2A Agonist 25CN-NBOH in Mice". Frontiers in Pharmacology 9: 17. 2018. doi:10.3389/fphar.2018.00017. PMID 29467649.
- ↑ "Cortical Correlates of Psychedelic-Induced Shaking Behavior Revealed by Voltage Imaging" (in en). International Journal of Molecular Sciences 24 (11): 9463. May 2023. doi:10.3390/ijms24119463. ISSN 1422-0067. PMID 37298417.
- ↑ "5-HT2 Receptor Regulation of Mitochondrial Genes: Unexpected Pharmacological Effects of Agonists and Antagonists". The Journal of Pharmacology and Experimental Therapeutics 357 (1): 1–9. April 2016. doi:10.1124/jpet.115.228395. PMID 26787771.
- ↑ "The 5-hydroxytryptamine 2A receptor agonists DOI and 25CN-NBOH decrease marble burying and reverse 8-OH-DPAT-induced deficit in spontaneous alternation". Neuropharmacology 183: 107838. February 2021. doi:10.1016/j.neuropharm.2019.107838. PMID 31693871.
- ↑ "Risperidone Treatment after Transient Ischemia Induces Hypothermia and Provides Neuroprotection in the Gerbil Hippocampus by Decreasing Oxidative Stress". International Journal of Molecular Sciences 20 (18): 4621. September 2019. doi:10.3390/ijms20184621. PMID 31540405.
- ↑ "Acute serotonin 2A receptor activation impairs behavioral flexibility in mice". Behavioural Brain Research 395: 112861. October 2020. doi:10.1016/j.bbr.2020.112861. PMID 32814148.
- ↑ "Serotonin Receptor 2A Activation Promotes Evolutionarily Relevant Basal Progenitor Proliferation in the Developing Neocortex". Neuron 108 (6): 1113–1129.e6. December 2020. doi:10.1016/j.neuron.2020.09.034. PMID 33080227.
- ↑ "Acute Lysergic Acid Diethylamide Does Not Influence Reward-Driven Decision Making of C57BL/6 Mice in the Iowa Gambling Task" (in English). Frontiers in Pharmacology 11: 602770. 2020. doi:10.3389/fphar.2020.602770. PMID 33343373.
- ↑ "Gs signaling pathway distinguishes hallucinogenic and nonhallucinogenic 5-HT2AR agonists induced head twitch response in mice". Biochemical and Biophysical Research Communications 598: 20–25. April 2022. doi:10.1016/j.bbrc.2022.01.113. PMID 35149433.
- ↑ "Serotonin Receptor 5-HT2A Regulates TrkB Receptor Function in Heteroreceptor Complexes". Cells 11 (15): 2384. August 2022. doi:10.3390/cells11152384. PMID 35954229.
- ↑ "The Chronic Treatment With 5-HT2A Receptor Agonists Affects the Behavior and the BDNF System in Mice". Neurochemical Research 45 (12): 3059–3075. December 2020. doi:10.1007/s11064-020-03153-5. PMID 33095437.
- ↑ "The selective 5-HT2A receptor agonist 25CN-NBOH does not affect reversal learning in mice". Behavioural Pharmacology 32 (5): 448–452. August 2021. doi:10.1097/FBP.0000000000000626. PMID 33595957.
- ↑ "Regulatory mechanism of CCN2 production by serotonin (5-HT) via 5-HT2A and 5-HT2B receptors in chondrocytes". PLOS ONE 12 (11): e0188014. 2017-11-16. doi:10.1371/journal.pone.0188014. PMID 29145495. Bibcode: 2017PLoSO..1288014H.
- ↑ "A Complex Impact of Systemically Administered 5-HT2A Receptor Ligands on Conditioned Fear". The International Journal of Neuropsychopharmacology 24 (9): 749–757. September 2021. doi:10.1093/ijnp/pyab040. PMID 34228806.
- ↑ "A quantitative method for the selective 5-HT2A agonist 25CN-NBOH in rat plasma and brain". Journal of Pharmaceutical and Biomedical Analysis 199: 114016. May 2021. doi:10.1016/j.jpba.2021.114016. PMID 33784574.
- ↑ "25CN-NBOH: A Selective Agonist for in vitro and in vivo Investigations of the Serotonin 2A Receptor". ChemMedChem 16 (21): 3263–3270. November 2021. doi:10.1002/cmdc.202100395. PMID 34288515.
- ↑ "Comparisons of hallucinogenic phenylisopropylamine binding affinities at cloned human 5-HT2A, -HT(2B) and 5-HT2C receptors". Naunyn-Schmiedeberg's Archives of Pharmacology 359 (1): 1–6. January 1999. doi:10.1007/PL00005315. PMID 9933142.
- ↑ "Extensive rigid analogue design maps the binding conformation of potent N-benzylphenethylamine 5-HT2A serotonin receptor agonist ligands". ACS Chemical Neuroscience 4 (1): 96–109. January 2013. doi:10.1021/cn3000668. PMID 23336049.
- ↑ "A Magyarországon megjelent, a Kábítószer és Kábítószer-függőség Európai Megfigyelő Központjának Korai Jelzőrendszerébe (EMCDDA EWS) 2005 óta bejelentett ellenőrzött anyagok büntetőjogi vonatkozású besorolása" (in Hungarian). September 2015. http://www.daath.hu/incoming/designer_jogi_lista_20150903_BSZKI_Daath_kieg.pdf.
- ↑ "The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014" (in en). http://www.legislation.gov.uk/uksi/2014/1106/made.
 |