Chemistry:Nefopam
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | nefopam medisol |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | intramuscular, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Low[1] |
| Protein binding | 70–75% (mean 73%)[1][2] |
| Metabolism | Liver (N-demethylation, others)[1] |
| Metabolites | Desmethylnefopam, others[1] |
| Elimination half-life | Nefopam: 3–8 hours[1] Desmethylnefopam: 10–15 hours[1] |
| Excretion | Urine: 79.3%[1] Feces: 13.4%[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| Chemical and physical data | |
| Formula | C17H19NO |
| Molar mass | 253.345 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Nefopam, sold under the brand name Acupan among others, is a centrally acting, non-opioid painkilling medication, that is primarily used to treat moderate to severe pain.[3]
Nefopam acts in the brain and spinal cord to relieve pain via novel mechanisms: antinociceptive effects from triple monoamine reuptake inhibition, and antihyperalgesic activity through modulation of glutamatergic transmission.[4]
Medical uses
Nefopam is effective for prevention of shivering during surgery or recovery from surgery.[5][6] Nefopam was significantly more effective than aspirin as an analgesic in one clinical trial,[7] although with a greater incidence of side effects such as sweating, dizziness and nausea, especially at higher doses.[8][9] The estimated relative potency of nefopam to morphine indicates that 20 mg of nefopam HCl is the approximate analgesic equal of 12 mg of morphine with comparable analgesic efficacy to morphine,[10][11][12] or oxycodone,[13] while nefopam tends to produce fewer side effects, does not produce respiratory depression,[14] and has much less abuse potential, and so is useful either as an alternative to opioid analgesics, or as an adjunctive treatment for use alongside opioids or other types of analgesics.[12][15] Nefopam is also used to treat severe hiccups.[16] Use of nefopam is also used for the manufacture of a medicament for the treatment of an affective disorder and attention-deficit disorder.[17]
Contraindications
Nefopam is contraindicated in people with convulsive disorders, those that have received treatment with irreversible monoamine oxidase inhibitors such as phenelzine, tranylcypromine or isocarboxazid within the past 30 days and those with myocardial infarction pain, mostly due to a lack of safety data in these conditions.[18]
Side effects
Common side effects include nausea, nervousness, dry mouth, light-headedness and urinary retention.[18] Less common side effects include vomiting, blurred vision, drowsiness, sweating, insomnia, headache, confusion, hallucinations, tachycardia, aggravation of angina and rarely a temporary and benign pink discolouration of the skin or erythema multiforme.[18]
Overdose
Overdose and death have been reported with nefopam.[19] Overdose usually manifests with convulsions, hallucinations, tachycardia, and hyperdynamic circulation.[18] Treatment is usually supportive, managing cardiovascular complications with beta blockers and limiting absorption with activated charcoal.[18]
Interactions
It has additive anticholinergic and sympathomimetic effects with other agents with these properties.[18] Its use should be avoided in people receiving some types of antidepressants (tricyclic antidepressants or monoamine oxidase inhibitors) as there is the potential for serotonin syndrome or hypertensive crises to result.[18]
Pharmacology
| Site | Ki (nM) |
|---|---|
| SERT | 29 |
| NET | 33 |
| DAT | 531 |
| 5-HT2A | 1,685 |
| 5-HT2B | 330 |
| 5-HT2C | 56 |
The mechanism of action of nefopam and its analgesic effects are not well understood, although inhibition of the reuptake of serotonin, norepinephrine, and to a lesser extent dopamine (that is, acting as an SNDRI) is thought to be involved.[20][4] It also reduces glutamate signaling via modulating sodium and calcium channels.[21][4]
Pharmacokinetics
The absolute bioavailability of nefopam is low.[1] It is reported to achieve therapeutic plasma concentrations between 49 and 183 nM.[22] The drug is approximately 73% protein-bound across a plasma range of 7 to 226 ng/mL (28–892 nM).[1] The metabolism of nefopam is hepatic, by N-demethylation and via other routes.[1] Its terminal half-life is 3 to 8 hours, while that of its active metabolite, desmethylnefopam, is 10 to 15 hours.[1] It is eliminated mostly in urine, and to a lesser extent in feces.[1]
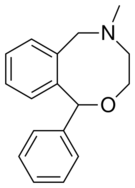
Chemistry
Nefopam is a cyclized analogue of orphenadrine, diphenhydramine, and tofenacin, with each of these compounds different from one another only by the presence of one or two carbons.[23][24][25] The ring system of nefopam is a benzoxazocine system.[23][26]
Society and culture
Recreational use
Recreational use of nefopam has rarely been reported,[19] and is far less common than with opioid analgesics.[27]
Names
In the 1960s, when it was first developed, it had the generic name fenazoxine.[21]
See also
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 "Pharmacokinetics, metabolism, and excretion of nefopam, a dual reuptake inhibitor in healthy male volunteers". Xenobiotica; the Fate of Foreign Compounds in Biological Systems 46 (11): 1001–16. November 2016. doi:10.3109/00498254.2015.1136989. PMID 26796604.
- ↑ Drug Dosage in Renal Insufficiency. Springer Science & Business Media. 6 December 2012. pp. 407–. ISBN 978-94-011-3804-8. https://books.google.com/books?id=OavnCAAAQBAJ&pg=PA407.
- ↑ "Nefopam hydrochloride". London, UK: Pharmaceutical Press. 27 October 2016. https://www.medicinescomplete.com/mc/martindale/current/2674-m.htm.
- ↑ 4.0 4.1 4.2 "Nefopam analgesia and its role in multimodal analgesia: A review of preclinical and clinical studies". Clinical and Experimental Pharmacology & Physiology 43 (1): 3–12. January 2016. doi:10.1111/1440-1681.12506. PMID 26475417.
- ↑ "Comparative effectiveness of pharmacologic interventions to prevent shivering after surgery: a network meta-analysis". Minerva Anestesiologica 85 (1): 60–70. January 2019. doi:10.23736/S0375-9393.18.12813-6. PMID 30226340.
- ↑ "Nefopam, a nonsedative benzoxazocine analgesic, selectively reduces the shivering threshold in unanesthetized subjects". Anesthesiology 100 (1): 37–43. January 2004. doi:10.1097/00000542-200401000-00010. PMID 14695722.
- ↑ "Nefopam hydrochloride: new analgesic agent". The Journal of International Medical Research 4 (2): 138–43. 1976. doi:10.1177/030006057600400211. PMID 799984.
- ↑ "The clinical analgesic efficacy of oral nefopam hydrochloride". Journal of Clinical Pharmacology 19 (7): 395–402. July 1979. doi:10.1002/j.1552-4604.1979.tb02498.x. PMID 479385.
- ↑ "Adverse reactions associated with nefopam". The New Zealand Medical Journal 108 (1008): 382–4. September 1995. PMID 7566787.
- ↑ "Nefopam and morphine in man". Clinical Pharmacology and Therapeutics 18 (5 Pt 1): 530–4. November 1975. doi:10.1002/cpt1975185part1530. PMID 1102231.
- ↑ "Nefopam in postoperative pain". British Journal of Anaesthesia 51 (10): 961–5. October 1979. doi:10.1093/bja/51.10.961. PMID 391253.
- ↑ 12.0 12.1 "Nefopam: a review of its pharmacological properties and therapeutic efficacy". Drugs 19 (4): 249–67. April 1980. doi:10.2165/00003495-198019040-00001. PMID 6991238.
- ↑ "Comparison of the analgesic dose-effect relationships of nefopam and oxycodone in postoperative pain". Acta Anaesthesiologica Scandinavica 23 (6): 555–60. December 1979. doi:10.1111/j.1399-6576.1979.tb01486.x. PMID 397711.
- ↑ "Respiratory effects of nefopam". Clinical Pharmacology and Therapeutics 18 (2): 175–9. August 1975. doi:10.1002/cpt1975182175. PMID 1097153.
- ↑ "Nefopam and ketamine comparably enhance postoperative analgesia". Anesthesia and Analgesia 100 (1): 169–74. January 2005. doi:10.1213/01.ANE.0000138037.19757.ED. PMID 15616073.
- ↑ "Nefopam for severe hiccups". The New England Journal of Medicine 343 (26): 1973–4. December 2000. doi:10.1056/nejm200012283432619. PMID 11186682.
- ↑ , Michael Harvey & Robin Mark Bannister"Use of nefopam for the treatment of affective disorders" patent WO2007012870A2, issued 2007-02-01
- ↑ 18.0 18.1 18.2 18.3 18.4 18.5 18.6 "Data Sheet ACUPAN™ Nefopam hydrochloride 30 mg tablets 20 mg intramuscular injection". Medsafe New Zealand. iNova Pharmaceuticals (New Zealand) Limited. 3 September 2007. http://www.medsafe.govt.nz/profs/datasheet/a/acupantabinj.pdf.
- ↑ 19.0 19.1 "[Chronic abuse of the analgesic nefopam (Acupan)]" (in French). Journal de Toxicologie Clinique et Expérimentale 7 (5): 343–6. September 1987. PMID 3448182.
- ↑ "New Zealand Data Sheet Acupan(TM)". Medsafe. New Zealand The Ministry of Health. 17 May 2017. http://www.medsafe.govt.nz/profs/Datasheet/a/acupantabinj.pdf.
- ↑ 21.0 21.1 "Rediscovery of nefopam for the treatment of neuropathic pain". The Korean Journal of Pain 27 (2): 103–11. April 2014. doi:10.3344/kjp.2014.27.2.103. PMID 24748937.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid22711801 - ↑ 23.0 23.1 The Practice of Medicinal Chemistry. Elsevier Science. 1 July 2015. pp. 250–251. ISBN 978-0-12-417213-5. https://books.google.com/books?id=dtScBAAAQBAJ&pg=PA250.
- ↑ Drug Discovery: A History. John Wiley & Sons. 23 June 2005. pp. 405–. ISBN 978-0-471-89979-2. https://books.google.com/books?id=Cb6BOkj9fK4C&pg=PA405.
- ↑ Chemogenomics in Drug Discovery: A Medicinal Chemistry Perspective. John Wiley & Sons. 6 March 2006. pp. 54–. ISBN 978-3-527-60402-9. https://books.google.com/books?id=oxvYXJSCImUC&pg=PA54.
- ↑ Therapeutic Hypothermia. CRC Press. 2014. pp. 176–. https://books.google.com/books?id=lpETxii5bjQC&pg=PA176.
- ↑ "Fatal overdosage with nefopam (Acupan)". Journal of Analytical Toxicology 26 (4): 239–43. May 2002. doi:10.1093/jat/26.4.239. PMID 12054367.
 |

