Chemistry:Butylone
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | oral, intravenous, insufflation |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C12H15NO3 |
| Molar mass | 221.256 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Butylone, also known as β-keto-N-methylbenzodioxolylbutanamine (βk-MBDB), is an entactogen, psychedelic, and stimulant psychoactive drug of the phenethylamine chemical class. It is the β-keto (substituted cathinone) analogue of MBDB and the substituted methylenedioxyphenethylamine analogue of buphedrone.
History
Butylone was first synthesized by Koeppe, Ludwig and Zeile which is mentioned in their 1967 paper. It remained an obscure product of academia until 2005 when it was sold as a designer drug.[1] Butylone shares the same relationship to MBDB as methylone does to MDMA ("Ecstasy"). Formal research on this chemical was first conducted in 2009, when it was shown to be metabolised in a similar manner to related drugs like methylone.[2]
Synthesis
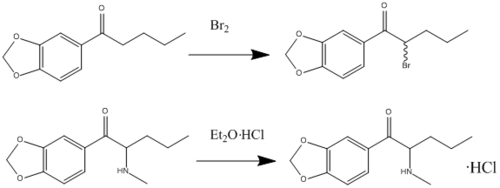
Butylone can be synthesized in a laboratory via the following route: 3,4-methylenedioxybutyrophenone dissolved in dichloromethane to bromine gives 3′,4′-methylenedioxy-2-bromobutyrophenone. This product was then dissolved in dichloromethane and added to an aqueous solution of methylamine (40%). HCl was then added. The aqueous layer was removed and made alkaline by using sodium bicarbonate. For the extraction of the amine ether was used. To get butylone a drop of ether and HCl solution was added.[3]
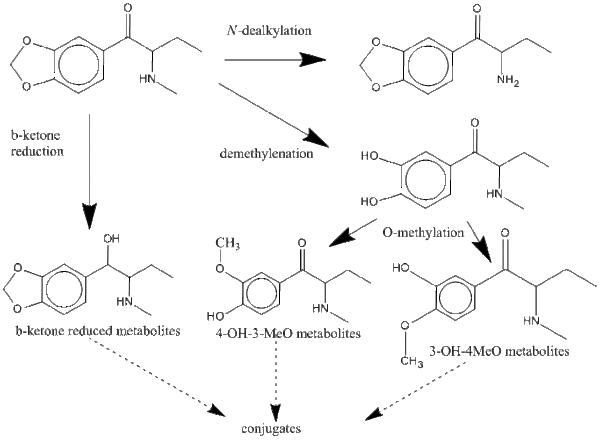
Metabolism
There are three major metabolic pathways of bk-MBDB as shown in the figure. As result of demethylenation followed by O-methylation bk-MBDB metabolises into 4-OH-3-MeO and 3-OH-4-MeO metabolites in human urine. The second pathway is a β-ketone reduction into β-ketone reduced metabolites. The third pathway is a N-dealkylation into N-dealkyl metabolites. The first two pathways occur more than pathway three. The most common metabolite is the 4-OH-3-MeO metabolite. The metabolites containing a hydroxyl-group would be excreted as their conjugates in urine.[4]
Mechanism of action
Butylone acts in a similar way as MDMA and Methylone, it causes an increase in extracellular monoamine levels.[5][6][3]
The following tables lists the half maximal inhibitory and half maximal effective concentrations for norepinephrine, dopamine and serotonin receptors, respectively.[7]
| NET | 2.02 (1.5–2.7) |
|---|---|
| DAT | 2.90 (2.5–3.4) |
| SERT | 6.22 (4.3–9.0) |
| DAT | >100 |
|---|---|
| SERT | 5.5 (1.8–17) |
Drug prohibition laws
China
As of October 2015 Butylone is a controlled substance in China.[8]
Sweden
Sveriges riksdag added butylone to schedule I ("substances, plant materials and fungi which normally do not have medical use") as narcotics in Sweden as of Feb 1, 2010, published by Medical Products Agency in their regulation LVFS 2022:48 listed as Butylon, 1-(1,3-bensodioxol-5-yl)-2-(metylamino)butan-1-on.[9]
United States
Butylone is also a Schedule I controlled substance under the Controlled Substances Act in the United States.
See also
References
- ↑ "年度買い上げ違法ドラッグ製品から検出されたデザイナードラッグ成分のNMRを中心とした分析" (in ja). Yakugaku Zasshi 128 (10): 1499–1505. October 2008. doi:10.1248/yakushi.128.1499. PMID 18827471.
- ↑ "Determination of the metabolites of the new designer drugs bk-MBDB and bk-MDEA in human urine". Forensic Science International 188 (1–3): 131–139. July 2009. doi:10.1016/j.forsciint.2009.04.001. PMID 19406592.
- ↑ 3.0 3.1 "Comparative neuropharmacology of three psychostimulant cathinone derivatives: butylone, mephedrone and methylone". British Journal of Pharmacology 167 (2): 407–420. September 2012. doi:10.1111/j.1476-5381.2012.01998.x. PMID 22509960.
- ↑ "The toxicology of bath salts: a review of synthetic cathinones". Journal of Medical Toxicology 8 (1): 33–42. March 2012. doi:10.1007/s13181-011-0193-z. PMID 22108839.
- ↑ "Substituted methcathinones differ in transporter and receptor interactions". Biochemical Pharmacology 85 (12): 1803–1815. June 2013. doi:10.1016/j.bcp.2013.04.004. PMID 23583454.
- ↑ "The synthetic cathinones, butylone and pentylone, are stimulants that act as dopamine transporter blockers but 5-HT transporter substrates". Psychopharmacology 236 (3): 953–962. March 2019. doi:10.1007/s00213-018-5075-5. PMID 30345459.
- ↑ "Pharmacological characterization of designer cathinones in vitro". British Journal of Pharmacology 168 (2): 458–470. January 2013. doi:10.1111/j.1476-5381.2012.02145.x. PMID 22897747.
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in zh). China Food and Drug Administration. 27 September 2015. http://www.sfda.gov.cn/WS01/CL0056/130753.html.
- ↑ Christina Rångemark Åkerman (29 July 2022). "Föreskrifter (HSLF-FS 2022:48) om ändring i Läkemedelsverkets föreskrifter (LVFS 2011:10) om förteckningar över narkotika" (in sv). LVFS. https://www.lakemedelsverket.se/sv/lagar-och-regler/foreskrifter/2022-48.
External links
 |