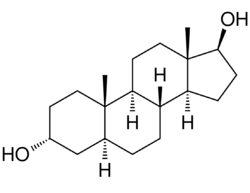
Chemistry:3α-Androstanediol
| |
| Names | |
|---|---|
| IUPAC name
5α-Androstane-3α,17β-diol
| |
| Systematic IUPAC name
(1S,3aS,3bR,5aS,7R,9aS,9bS,11aS)-9a,11a-Dimethylhexadecahydro-1H-cyclopenta[a]phenanthrene-1,7-diol | |
| Other names
Hombreol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C19H32O2 | |
| Molar mass | 292.463 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
3α-Androstanediol also known as 5α-androstane-3α,17β-diol and sometimes shortened in the literature to 3α-diol, is an endogenous steroid hormone and neurosteroid and a metabolite of androgens like dihydrotestosterone (DHT).[1][2][3]
Biological activity
3α-Androstanediol is an inhibitory androstane neurosteroid and weak androgen and estrogen.[1][2][3]
As a neurosteroid, it acts as a potent positive allosteric modulator of the GABAA receptor,[4] and has been found to have rewarding,[5][6] anxiolytic,[7] pro-sexual,[8] and anticonvulsant effects.[9][10] As androgens such as testosterone and DHT are known to have many of the same effects as 3α-diol and are converted into it in vivo, it is thought that this compound may in part be responsible for said effects.[5][6][7][10]
Relative to its isomer 3β-androstanediol, which is a potent estrogen, 3α-androstanediol has substantially lower, though still significant affinity for the estrogen receptors, with a several-fold preference for ERβ over ERα.[11][12] It has approximately 0.07% and 0.3% of the affinity of estradiol at the ERα and ERβ, respectively.[13]
Biochemistry
3α-Androstanediol shows high affinity for sex hormone-binding globulin (SHBG), similar to that of testosterone.[14]
Chemistry
3α-Androstanediol, also known as 5α-androstane-3α,17β-diol, is a naturally occurring androstane steroid and a structural analogue of DHT (5α-androstan-17β-ol-3-one). A notable positional isomer of 3α-androstanediol is 3β-androstanediol.
An orally active synthetic analogue of 3α-androstanediol, 17α-ethynyl-3α-androstanediol (HE-3235, Apoptone), was formerly under investigation for the treatment of prostate cancer and breast cancer.[15]
References
- ↑ 1.0 1.1 Reddy DS (2010). "Neurosteroids". Sex Differences in the Human Brain, their Underpinnings and Implications. Progress in Brain Research. 186. 113–37. doi:10.1016/B978-0-444-53630-3.00008-7. ISBN 9780444536303.
- ↑ 2.0 2.1 "Steroid 5alpha-reductases and 3alpha-hydroxysteroid dehydrogenases: key enzymes in androgen metabolism". Best Pract. Res. Clin. Endocrinol. Metab. 15 (1): 79–94. March 2001. doi:10.1053/beem.2001.0120. PMID 11469812.
- ↑ 3.0 3.1 "Identification of the molecular switch that regulates access of 5alpha-DHT to the androgen receptor". Mol. Cell. Endocrinol. 265-266: 77–82. February 2007. doi:10.1016/j.mce.2006.12.007. PMID 17223255.
- ↑ "The testosterone-derived neurosteroid androstanediol is a positive allosteric modulator of GABAA receptors". J. Pharmacol. Exp. Ther. 334 (3): 1031–41. September 2010. doi:10.1124/jpet.110.169854. PMID 20551294.
- ↑ 5.0 5.1 Frye CA (February 2007). "Some rewarding effects of androgens may be mediated by actions of its 5alpha-reduced metabolite 3alpha-androstanediol". Pharmacol. Biochem. Behav. 86 (2): 354–67. doi:10.1016/j.pbb.2006.10.003. PMID 17112575.
- ↑ 6.0 6.1 "The testosterone metabolite and neurosteroid 3alpha-androstanediol may mediate the effects of testosterone on conditioned place preference". Brain Res. Brain Res. Rev. 37 (1–3): 162–71. November 2001. doi:10.1016/s0165-0173(01)00116-3. PMID 11744084.
- ↑ 7.0 7.1 "Anxiolytic-like actions of testosterone in the burying behavior test: role of androgen and GABA-benzodiazepine receptors". Psychoneuroendocrinology 30 (8): 762–70. September 2005. doi:10.1016/j.psyneuen.2005.03.006. PMID 15919582.
- ↑ "The testosterone metabolite 3α-diol enhances female rat sexual motivation when infused in the nucleus accumbens shell". J Sex Med 7 (11): 3598–609. November 2010. doi:10.1111/j.1743-6109.2010.01937.x. PMID 20646182.
- ↑ Reddy DS (March 2004). "Anticonvulsant activity of the testosterone-derived neurosteroid 3alpha-androstanediol". NeuroReport 15 (3): 515–8. doi:10.1097/00001756-200403010-00026. PMID 15094514.
- ↑ 10.0 10.1 Reddy DS (2004). "Testosterone modulation of seizure susceptibility is mediated by neurosteroids 3alpha-androstanediol and 17beta-estradiol". Neuroscience 129 (1): 195–207. doi:10.1016/j.neuroscience.2004.08.002. PMID 15489042.
- ↑ "Recent insights into the origins of adrenal and sex steroid receptors". J. Mol. Endocrinol. 28 (3): 149–52. 2002. doi:10.1677/jme.0.0280149. PMID 12063181. https://cloudfront.escholarship.org/dist/prd/content/qt8qd4j1k2/qt8qd4j1k2.pdf.
- ↑ Kuiper, George G. J. M.; Carlsson, Bo; Grandien, Kaj; Enmark, Eva; Häggblad, Johan; Nilsson, Stefan; Gustafsson, Jan-Åke (1997). "Comparison of the Ligand Binding Specificity and Transcript Tissue Distribution of Estrogen Receptors α and β". Endocrinology 138 (3): 863–870. doi:10.1210/endo.138.3.4979. ISSN 0013-7227. PMID 9048584.
- ↑ "Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta". Endocrinology 138 (3): 863–70. 1997. doi:10.1210/endo.138.3.4979. PMID 9048584.
- ↑ "Human sex hormone-binding globulin binding affinities of 125 structurally diverse chemicals and comparison with their binding to androgen receptor, estrogen receptor, and α-fetoprotein". Toxicol. Sci. 143 (2): 333–48. February 2015. doi:10.1093/toxsci/kfu231. PMID 25349334.
- ↑ "17α-alkynyl 3α, 17β-androstanediol non-clinical and clinical pharmacology, pharmacokinetics and metabolism". Invest New Drugs 30 (1): 59–78. 2012. doi:10.1007/s10637-010-9517-0. PMID 20814732.
 |

