Chemistry:Fenfluramine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Seizures: Fintepla Appetite suppressant: Pondimin, Ponderax, Ponderal, others |
| Other names | ZX008; 3-Trifluoromethyl-N-ethylamphetamine |
| AHFS/Drugs.com | Professional Drug Facts |
| MedlinePlus | a620045 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 13–30 hours[3] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C12H16F3N |
| Molar mass | 231.262 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Fenfluramine, sold under the brand name Fintepla, is a serotonergic medication used for the treatment of seizures associated with Dravet syndrome and Lennox–Gastaut syndrome.[4][5][1] It was formerly used as an appetite suppressant in the treatment of obesity, but was discontinued for this use due to cardiovascular toxicity before being repurposed for new indications.[6][7] Fenfluramine was used for weight loss both alone under the brand name Pondimin and in combination with phentermine.[6][8]
Side effects of fenfluramine in people treated for seizures include decreased appetite, somnolence, sedation, lethargy, diarrhea, constipation, abnormal echocardiogram, fatigue, malaise, asthenia, ataxia, balance disorder, gait disturbance, increased blood pressure, drooling, excessive salivation, fever, upper respiratory tract infection, vomiting, appetite loss, weight loss, falls, and status epilepticus.[4] Fenfluramine acts as a serotonin releasing agent, agonist of the serotonin 5-HT2 receptors, and σ1 receptor positive modulator.[9][10][11] Its mechanism of action in the treatment of seizures is unknown,[4] but may involve increased activation of certain serotonin receptors and the σ1 receptor.[10][7][12]
Fenfluramine was developed in the early 1960s and was first introduced for medical use as an appetite suppressant in France in 1963 followed by approval in the United States in 1973.[6] In the 1990s, fenfluramine came to be associated with cardiovascular toxicity, and because of this, was withdrawn from the United States market in 1997.[6][13] Subsequently, it was repurposed for the treatment of seizures and was reintroduced in the United States and the European Union in 2020.[5][1][7] Fenfluramine was previously a schedule IV controlled substance in the United States.[5] However, the substance has since no-longer been subject to control pursuant to rule-making issued on 23 December 2022.[14]
Medical uses
Seizures
Fenfluramine is indicated for the treatment of seizures associated with Dravet syndrome and Lennox–Gastaut syndrome in people age two and older.[4][5][1]
Dravet syndrome is a life-threatening, rare and chronic form of epilepsy.[5] It is often characterized by severe and unrelenting seizures despite medical treatment.[5]
Obesity
Fenfluramine was formerly used as an appetite suppressant in the treatment of obesity, but was withdrawn for this use due to cardiovascular toxicity.[6]
Adverse effects
The most common adverse reactions in people with seizures include decreased appetite; drowsiness, sedation and lethargy; diarrhea; constipation; abnormal echocardiogram; fatigue or lack of energy; ataxia (lack of coordination), balance disorder, gait disturbance (trouble with walking); increased blood pressure; drooling, salivary hypersecretion (saliva overproduction); pyrexia (fever); upper respiratory tract infection; vomiting; decreased weight; risk of falls; and status epilepticus.[5]
The U.S. Food and Drug Administration (FDA) fenfluramine labeling includes a boxed warning stating the drug is associated with valvular heart disease (VHD) and pulmonary arterial hypertension (PAH).[5] Because of the risks of VHD and PAH, fenfluramine is available only through a restricted drug distribution program, under a risk evaluation and mitigation strategy (REMS).[5] The fenfluramine REMS requires health care professionals who prescribe fenfluramine and pharmacies that dispense fenfluramine to be specially certified in the fenfluramine REMS and that patients be enrolled in the REMS.[5] As part of the REMS requirements, prescribers and patients must adhere to the required cardiac monitoring with echocardiograms to receive fenfluramine.[5]
At higher therapeutic doses, headache, diarrhea, dizziness, dry mouth, erectile dysfunction, anxiety, insomnia, irritability, lethargy, and CNS stimulation have been reported with fenfluramine.[3]
There have been reports associating chronic fenfluramine treatment with emotional instability, cognitive deficits, depression, psychosis, exacerbation of pre-existing psychosis (schizophrenia), and sleep disturbances.[3][15] It has been suggested that some of these effects may be mediated by serotonergic neurotoxicity/depletion of serotonin with chronic administration and/or activation of serotonin 5-HT2A receptors.[15][16][17][18]
Heart valve disease
The distinctive valvular abnormality seen with fenfluramine is a thickening of the leaflet and chordae tendineae. One mechanism used to explain this phenomenon involves heart valve serotonin receptors, which are thought to help regulate growth. Since fenfluramine and its active metabolite norfenfluramine stimulate serotonin receptors, this may have led to the valvular abnormalities found in patients using fenfluramine. In particular norfenfluramine is a potent inhibitor of the re-uptake of 5-HT into nerve terminals.[19] Fenfluramine and its active metabolite norfenfluramine affect the 5-HT2B receptors, which are plentiful in human cardiac valves. The suggested mechanism by which fenfluramine causes damage is through over or inappropriate stimulation of these receptors leading to inappropriate valve cell division. Supporting this idea is the fact that this valve abnormality has also occurred in patients using other drugs that act on 5-HT2B receptors.[20][21]
According to a study of 5,743 former users conducted by a plaintiff's expert cardiologist, damage to the heart valve continued long after stopping the medication.[22] Of the users tested, 20% of women, and 12% of men were affected. For all ex-users, there was a 7-fold increase of chances of needing surgery for faulty heart valves caused by the drug.[22]
Overdose
In overdose, fenfluramine can cause serotonin syndrome and rapidly result in death.[6][23]
Pharmacology
Pharmacodynamics
Fenfluramine acts primarily as a serotonin releasing agent.[24][25] It increases the level of serotonin, a neurotransmitter that regulates mood, appetite and other functions.[24][25] Fenfluramine causes the release of serotonin by disrupting vesicular storage of the neurotransmitter, and reversing serotonin transporter function.[26] The drug also acts as a norepinephrine releasing agent to a lesser extent, particularly via its active metabolite norfenfluramine.[24][25] At high concentrations, norfenfluramine, though not fenfluramine, also acts as a dopamine releasing agent, and so fenfluramine may do this at very high doses as well.[24][25] In addition to monoamine release, while fenfluramine binds only very weakly to the serotonin 5-HT2 receptors, norfenfluramine binds to and activates the serotonin 5-HT2B and 5-HT2C receptors with high affinity and the serotonin 5-HT2A receptor with moderate affinity.[27][28] The result of the increased serotonergic and noradrenergic neurotransmission is a feeling of fullness and reduced appetite.
The combination of fenfluramine with phentermine, a norepinephrine–dopamine releasing agent acting primarily on norepinephrine, results in a well-balanced serotonin–norepinephrine releasing agent with weaker effects of dopamine release.[24][25]
| Drug | NE | DA | 5-HT | Type | Ref |
|---|---|---|---|---|---|
| D-Fenfluramine | 302 | >10,000 | 51.7 | SNRA | [29][24] |
| L-Fenfluramine | >10,000 | >10,000 | 147 | SRA | [24][30] |
| Norfenfluramine | 168–170 | 1,900–1,925 | 104 | SNRA | [24][25] |
| Phentermine | 39.4 | 262 | 3,511 | NDRA | [29] |
Fenfluramine was identified as a potent positive modulator of the σ1 receptor in 2020 and this action may be involved in its therapeutic benefits in the treatment of seizures.[10][11]
Pharmacokinetics
The elimination half-life of fenfluramine has been reported as ranging from 13 to 30 hours.[3] The mean elimination half-lives of its enantiomers have been found to be 19 hours for dexfenfluramine and 25 hours for levfenfluramine.[6] Norfenfluramine, the major active metabolite of fenfluramine, has an elimination half-life that is about 1.5 to 2 times as long as that of fenfluramine, with mean values of 34 hours for dexnorfenfluramine and 50 hours for levnorfenfluramine.[6]
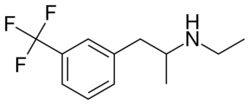
Chemistry
Fenfluramine is a substituted amphetamine and is also known as 3-trifluoromethyl-N-ethylamphetamine.[6] It is a racemic mixture of two enantiomers, dexfenfluramine and levofenfluramine.[6] Some analogues of fenfluramine include norfenfluramine, benfluorex, flucetorex, and fludorex.
History
Fenfluramine was developed in the early 1960s and was introduced in France in 1963.[6] Approximately 50 million Europeans were treated with fenfluramine for appetite suppression between 1963 and 1996.[6] Fenfluramine was approved in the United States in 1973.[6] The combination of fenfluramine and phentermine was proposed in 1984.[6] Approximately 5 million people in the United States were given fenfluramine or dexfenfluramine with or without phentermine between 1996 and 1998.[6]
In the early 1990s, French researchers reported an association of fenfluramine with primary pulmonary hypertension and dyspnea in a small sample of patients.[6] Fenfluramine was withdrawn from the U.S. market in 1997 after reports of heart valve disease[31][13] and continued findings of pulmonary hypertension, including a condition known as cardiac fibrosis.[32] It was subsequently withdrawn from other markets around the world. It was banned in India in 1998.[33]
Fenfluramine was an appetite suppressant which was used to treat obesity.[6] It was used both on its own and, in combination with phentermine, as part of the anti-obesity medication Fen-Phen.[6]
In June 2020, fenfluramine was approved for medical use in the United States with an indication to treat Dravet syndrome.[5][34]
The effectiveness of fenfluramine for the treatment of seizures associated with Dravet syndrome was demonstrated in two clinical studies in 202 subjects between ages two and eighteen.[5] The studies measured the change from baseline in the frequency of convulsive seizures.[5] In both studies, subjects treated with fenfluramine had significantly greater reductions in the frequency of convulsive seizures during the trials than subjects who received placebo (inactive treatment).[5] These reductions were seen within 3–4 weeks, and remained generally consistent over the 14- to 15-week treatment periods.[5]
The U.S. Food and Drug Administration (FDA) granted the application for fenfluramine priority review and orphan drug designations.[5][35][36] The FDA granted approval of Fintepla to Zogenix, Inc.[5]
On 15 October 2020, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Fintepla, intended for the treatment of seizures associated with Dravet syndrome.[37] Fenfluramine was approved for medical use in the European Union in December 2020.[1]
Society and culture
Legal status
Fenfluramine is a prescription medication in the US. Fenfluramine was removed from Schedule IV of the Controlled Substances Act in December 2022.[38]
Recreational use
Unlike various other amphetamine derivatives, fenfluramine is reported to be dysphoric, "unpleasantly lethargic", and non-addictive at therapeutic doses.[39] However, it has been reported to be used recreationally at high doses ranging between 80 and 400 mg, which have been described as producing euphoria, amphetamine-like effects, sedation, and hallucinogenic effects, along with anxiety, nausea, diarrhea, and sometimes panic attacks, as well as depressive symptoms once the drug had worn off.[39][40][41] At very high doses (e.g., 240 mg, or between 200 and 600 mg), fenfluramine induces a psychedelic state resembling that produced by lysergic acid diethylamide (LSD).[41][42] Indirect (via induction of serotonin release) and/or direct activation of the 5-HT2A receptor would be expected to be responsible for the psychedelic effects of the drug at sufficient doses.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Fintepla EPAR". 13 October 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/fintepla.
- ↑ "Fintepla Product information". https://ec.europa.eu/health/documents/community-register/html/h1491.htm.
- ↑ 3.0 3.1 3.2 3.3 Medical Toxicology. Lippincott Williams & Wilkins. 2004. pp. 874–. ISBN 978-0-7817-2845-4. https://books.google.com/books?id=BfdighlyGiwC&pg=PA874.
- ↑ 4.0 4.1 4.2 4.3 "FINTEPLA (fenfluramine) oral solution". Zogenix Inc.. U.S. Food and Drug Administration. March 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/212102s003lbl.pdf.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 5.16 5.17 "FDA Approves New Therapy for Dravet Syndrome". U.S. Food and Drug Administration (FDA) (Press release). 25 June 2020. Retrieved 25 June 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 6.12 6.13 6.14 6.15 6.16 6.17 Medical Toxicology of Drug Abuse: Synthesized Chemicals and Psychoactive Plants. John Wiley & Sons. 3 February 2012. pp. 255–262. ISBN 978-1-118-10605-1. https://books.google.com/books?id=9JLiJcjdqkcC&pg=PA255.
- ↑ 7.0 7.1 7.2 "Fenfluramine repurposing from weight loss to epilepsy: What we do and do not know". Pharmacol Ther 226: 107866. October 2021. doi:10.1016/j.pharmthera.2021.107866. PMID 33895186.
- ↑ Swiss Pharmaceutical Society (2000). Swiss Pharmaceutical Society. ed. Index Nominum 2000: International Drug Directory. Taylor & Francis. pp. 431–432. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA431.
- ↑ "Neurochemical mechanisms of phentermine and fenfluramine: Therapeutic and adverse effects". Drug Development Research 51 (2): 52–65. 2000. doi:10.1002/1098-2299(200010)51:2<52::AID-DDR2>3.0.CO;2-H. ISSN 0272-4391.
- ↑ 10.0 10.1 10.2 "An Emerging Role for Sigma-1 Receptors in the Treatment of Developmental and Epileptic Encephalopathies". Int J Mol Sci 22 (16): 8416. August 2021. doi:10.3390/ijms22168416. PMID 34445144.
- ↑ 11.0 11.1 "Fenfluramine acts as a positive modulator of sigma-1 receptors". Epilepsy Behav 105: 106989. April 2020. doi:10.1016/j.yebeh.2020.106989. PMID 32169824.
- ↑ "Individualized treatment approaches: Fenfluramine, a novel antiepileptic medication for the treatment of seizures in Dravet syndrome". Epilepsy Behav 91: 99–102. February 2019. doi:10.1016/j.yebeh.2018.08.021. PMID 30269941.
- ↑ 13.0 13.1 "Appetite suppressants and valvular heart disease". The American Journal of the Medical Sciences 321 (4): 285–291. April 2001. doi:10.1097/00000441-200104000-00008. PMID 11307869.
- ↑ "Schedules of Controlled Substances: Removal of Fenfluramine From Control". U.S. Federal Register. 23 December 2022. https://www.federalregister.gov/documents/2022/12/23/2022-27400/schedules-of-controlled-substances-removal-of-fenfluramine-from-control.
- ↑ 15.0 15.1 Drug Injury: Liability, Analysis, and Prevention. Lawyers & Judges Publishing Company. 2005. pp. 276–. ISBN 978-0-913875-27-8. https://books.google.com/books?id=EB00rD8AqaYC&pg=PA276.
- ↑ Integrating the Neurobiology of Schizophrenia. Academic Press. 27 February 2007. pp. 142–. ISBN 978-0-08-047508-0. https://books.google.com/books?id=mksAKriZXuYC&pg=PA142.
- ↑ The Pharmacology of Corticotropin-releasing Factor (CRF). Effects on Sensorimotor Gating in the Rat. 2006. pp. 12–. ISBN 978-0-549-53661-1. https://books.google.com/books?id=kTADn_LMnZ4C&pg=PA12.[yes|permanent dead link|dead link}}]
- ↑ Handbook of the Behavioral Neurobiology of Serotonin. Academic Press. 30 December 2009. pp. 630–. ISBN 978-0-08-087817-1. https://books.google.com/books?id=aomaKqIE1jUC&pg=PA630.
- ↑ "Evidence that hypophagia induced by d-fenfluramine and d-norfenfluramine in the rat is mediated by 5-HT2C receptors". Neuropharmacology 41 (2): 200–9. 2001. doi:10.1016/s0028-3908(01)00063-6. PMID 11489456.
- ↑ "Drugs and valvular heart disease". The New England Journal of Medicine 356 (1): 6–9. January 2007. doi:10.1056/NEJMp068265. PMID 17202450.
- ↑ "Serotonergic drugs and valvular heart disease". Expert Opinion on Drug Safety 8 (3): 317–329. May 2009. doi:10.1517/14740330902931524. PMID 19505264.
- ↑ 22.0 22.1 "Valvular regurgitation and surgery associated with fenfluramine use: an analysis of 5743 individuals". BMC Medicine 6: 34. November 2008. doi:10.1186/1741-7015-6-34. PMID 18990200.
- ↑ Neuroleptic Malignant Syndrome and Related Conditions. American Psychiatric Pub. 20 May 2008. pp. 111–. ISBN 978-1-58562-744-8. https://books.google.com/books?id=POjw-pQcvW8C&pg=PA111.
- ↑ 24.0 24.1 24.2 24.3 24.4 24.5 24.6 24.7 Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid12649307 - ↑ 25.0 25.1 25.2 25.3 25.4 25.5 Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid12761331 - ↑ Molecular Neuropharmacology: A Foundation for Clinical Neuroscience. McGraw-Hill. 2001.
- ↑ "Dopamine/serotonin releasers as medications for stimulant addictions". Serotonin-dopamine Interaction: Experimental Evidence and Therapeutic Relevance. Elsevier. 2008. pp. 393–. ISBN 978-0-444-53235-0. https://books.google.com/books?id=mPkKtA15KM8C&pg=PA393.
- ↑ "Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine". Mol. Pharmacol. 57 (1): 75–81. 2000. PMID 10617681.
- ↑ 29.0 29.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid11071707 - ↑ "Therapeutic and adverse actions of serotonin transporter substrates". Pharmacol. Ther. 95 (1): 73–88. 2002. doi:10.1016/s0163-7258(02)00234-6. PMID 12163129.
- ↑ "Valvular heart disease associated with fenfluramine-phentermine". The New England Journal of Medicine 337 (9): 581–588. August 1997. doi:10.1056/NEJM199708283370901. PMID 9271479.
- ↑ "FDA Announces Withdrawal Fenfluramine and Dexfenfluramine (Fen-Phen)". U.S. Food and Drug Administration. 15 September 1997. https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm179871.htm.
- ↑ "Drugs banned in India". Central Drugs Standard Control Organization, Dte.GHS, Ministry of Health and Family Welfare, Government of India. http://cdsco.nic.in/html/drugsbanned.html.
- ↑ "FDA Approves FINTEPLA (fenfluramine) for the Treatment of Seizures Associated with Dravet Syndrome" (Press release). Zogenix Inc. 25 June 2020. Retrieved 25 June 2020 – via GlobeNewswire.
- ↑ "Fenfluramine Orphan Drug Designations and Approvals". 24 December 1999. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=414613.
- ↑ "Fenfluramine Orphan Drug Designations and Approvals". 24 December 1999. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=584217.
- ↑ "Fintepla: Pending EC decision". 16 October 2020. https://www.ema.europa.eu/en/medicines/human/summaries-opinion/fintepla. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Schedules of Controlled Substances: Removal of Fenfluramine from Control". DEA. December 23, 2022. https://www.federalregister.gov/documents/2022/12/23/2022-27400/schedules-of-controlled-substances-removal-of-fenfluramine-from-control.
- ↑ 39.0 39.1 Neurological Aspects of Substance Abuse. Butterworth-Heinemann. 2004. pp. 117–. ISBN 978-0-7506-7313-6. https://books.google.com/books?id=fOfxoQm_a7MC&pg=PA117.
- ↑ Competitive problems in the drug industry: hearings before Subcommittee on Monopoly and Anticompetitive Activities of the Select Committee on Small Business, United States Senate, Ninetieth Congress, first session. U.S. Government Printing Office. 1976. pp. 2–. https://books.google.com/books?id=Rs1GAQAAMAAJ&pg=RA2-PA56.
- ↑ 41.0 41.1 Drug Addiction II: Amphetamine, Psychotogen, and Marihuana Dependence. Springer Science & Business Media. 27 November 2013. pp. 258–. ISBN 978-3-642-66709-1. https://books.google.com/books?id=gb_uCAAAQBAJ&pg=PA258.
- ↑ Drug Addiction II: Amphetamine, Psychotogen, and Marihuana Dependence. Springer Science & Business Media. 27 November 2013. pp. 249–. ISBN 978-3-642-66709-1. https://books.google.com/books?id=gb_uCAAAQBAJ&pg=PA249.
Further reading
- "Long-term serotonin administration induces heart valve disease in rats". Circulation 111 (12): 1517–22. March 2005. doi:10.1161/01.CIR.0000159356.42064.48. PMID 15781732.
- "The synthesis and biological activity of pentafluorosulfanyl analogs of fluoxetine, fenfluramine, and norfenfluramine". Bioorganic & Medicinal Chemistry 15 (21): 6659–6666. November 2007. doi:10.1016/j.bmc.2007.08.012. PMID 17765553.
External links
 |

