Chemistry:Isoflurane
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Forane, others |
| AHFS/Drugs.com | FDA Professional Drug Information |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Inhalation |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
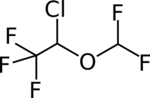
| Formula | C3H2ClF5O |
| Molar mass | 184.49 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Isoflurane, sold under the brand name Forane among others, is a general anesthetic.[3] It can be used to start or maintain anesthesia; however, other medications are often used to start anesthesia, due to airway irritation with isoflurane.[2][4] Isoflurane is given via inhalation.[3]
Side effects of isoflurane include a decreased ability to breathe (respiratory depression), low blood pressure, and an irregular heartbeat.[2] Serious side effects can include malignant hyperthermia or high blood potassium.[3] It should not be used in patients with a history of malignant hyperthermia in either themselves or their family members.[2] It is unknown if its use during pregnancy is safe for the fetus, but use during a cesarean section appears to be safe.[2][3] Isoflurane is a halogenated ether.[5]
Isoflurane was approved for medical use in the United States in 1979.[3][6] It is on the World Health Organization's List of Essential Medicines.[7][8]
Medical uses
Isoflurane is always administered in conjunction with air or pure oxygen. Often, nitrous oxide is also used. Although its physical properties imply that anaesthesia can be induced more rapidly than with halothane,[9] its pungency can irritate the respiratory system, negating any possible advantage conferred by its physical properties. Thus, it is mostly used in general anesthesia as a maintenance agent after induction of general anesthesia with an intravenous agent such as thiopentone or propofol.[10][11][12]
Mechanism of action
Similar to many general anesthetics, the exact mechanism of the action has not been clearly delineated.[13] Isoflurane reduces pain sensitivity (analgesia) and relaxes muscles. Isoflurane likely binds to GABA, glutamate and glycine receptors, but has different effects on each receptor. Isoflurane acts as a positive allosteric modulator of the GABAA receptor in electrophysiology studies of neurons and recombinant receptors.[14][15][16][17] It potentiates glycine receptor activity, which decreases motor function.[18] It inhibits receptor activity in the NMDA glutamate receptor subtypes. Isoflurane inhibits conduction in activated potassium channels.[19] Isoflurane also affects intracellular molecules. It inhibits plasma membrane calcium ATPases (PMCAs) which affects membrane fluidity by hindering the flow of Ca2+ (calcium ions) out across the membrane, this in turn affects neuron depolarization.[20][21] It binds to the D subunit of ATP synthase and NADH dehydrogenase.
General anaesthesia with isoflurane reduces plasma endocannabinoid AEA concentrations, and this could be a consequence of stress reduction after loss of consciousness.[22]
Adverse effects
Isoflurane can cause a sudden decrease in blood pressure due to dose-dependent peripheral vasodilation. This may be specially marked in hypovolemic patients.[12]
Animal studies have raised safety concerns of certain general anesthetics, in particular ketamine and isoflurane, in young children. The risk of neurodegeneration was increased in combination of these agents with nitrous oxide and benzodiazepines such as midazolam.[23] Whether these concerns occur in humans is unclear.[23]
Elderly
Biophysical studies using NMR spectroscopy has provided molecular details of how inhaled anesthetics interact with three amino acid residues (G29, A30 and I31) of amyloid beta peptide and induce aggregation.[24] This area is important as "some of the commonly used inhaled anesthetics may cause brain damage that accelerates the onset of Alzheimer's disease".[25]
Physical properties
| Molecular weight | 184.5g/mol[26] | ||
| Boiling point (at 1 atm): | 48.5 °C[26] | ||
| Density (at 25 °C): | 1.496 g/mL[26] | ||
| MAC : | 1.15 vol % | ||
| Vapor pressure: | 238 mmHg | 31.7 kPa | (at 20 °C) |
| 295 mmHg | 39.3 kPa | (at 25 °C) | |
| 367 mmHg | 48.9 kPa | (at 30 °C) | |
| 450 mmHg | 60.0 kPa | (at 35 °C)[26] | |
| Water solubility | 13.5 mM | (at 25 °C)[27] | |
| Blood:gas partition coefficient: | 1.4 | ||
| Oil:gas partition coefficient: | 98 |
It is administered as a racemic mixture of (R)- and (S)-optical isomers.[28] Isoflurane has a boiling point of 48.5 - 49 °C (119 - 120 °F).[26] It is non-combustible but can give off irritable and toxic fumes when exposed to flame.[26]
History
Together with enflurane and halothane, Isoflurane began to replace the flammable ethers used in the pioneer days of surgery; this shift began in the 1940s to the 1950s.[29] Its name comes from being a structural isomer of enflurane, hence they have the same empirical formula.[30]
Environment
The average lifetime of isoflurane in the atmosphere is 3.2 years, its global warming potential is 510 and the yearly emissions add up to 880 tons.[31]
Veterinary use
Isoflurane is frequently used for veterinary anaesthesia.[32][33]
References
- ↑ "Isoflurane Use During Pregnancy". 2 September 2020. https://www.drugs.com/pregnancy/isoflurane.html.
- ↑ 2.0 2.1 2.2 2.3 2.4 "Isoflurane 100% Inhalation Vapour, Liquid - Summary of Product Characteristics (SmPC)". 29 October 2019. https://www.medicines.org.uk/emc/product/9800/smpc.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 "Forane- isoflurane inhalant". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3d30eb8d-a62e-475f-926b-78ba63bee9c8.
- ↑ (in en) Nelson Textbook of Pediatrics (20 ed.). Elsevier Health Sciences. 2015. p. 420. ISBN 9780323263528. https://books.google.com/books?id=P9piCAAAQBAJ&pg=PA420.
- ↑ (in en) Essential Clinical Anesthesia Review: Keywords, Questions and Answers for the Boards. Cambridge University Press. 2015. p. 115. ISBN 9781107681309. https://books.google.com/books?id=VJzWBQAAQBAJ&pg=PA115.
- ↑ "Forane: FDA-Approved Drugs". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=017624.
- ↑ World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. 2021. WHO/MHP/HPS/EML/2021.02.
- ↑ Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. Lippincott Williams & Wilkins. 2005. p. 1156. ISBN 978-0-7817-5126-1. https://books.google.com/books?id=tndqYGPHQdEC&pg=PA1156.
- ↑ "Clinical experience with isoflurane (Forane): preliminary communication". British Journal of Anaesthesia 45 (7): 697–703. July 1973. doi:10.1093/bja/45.7.697. PMID 4730162.
- ↑ "The effects of isoflurane and propofol on intraoperative neurophysiological monitoring during spinal surgery". Journal of Clinical Monitoring and Computing 18 (4): 303–308. August 2004. doi:10.1007/s10877-005-5097-5. PMID 15779842.
- ↑ 12.0 12.1 "Isoflurane". StatPearls. 2022. https://www.ncbi.nlm.nih.gov/books/NBK532957/.
- ↑ "How does anesthesia work?". Scientific American. February 7, 2005. http://www.scientificamerican.com/article/how-does-anesthesia-work/.
- ↑ "Enhancement of gamma-aminobutyric acid-activated Cl- currents in cultured rat hippocampal neurones by three volatile anaesthetics". The Journal of Physiology 449: 279–293. April 1992. doi:10.1113/jphysiol.1992.sp019086. PMID 1326046.
- ↑ "Effects of temperature and volatile anesthetics on GABA(A) receptors". Anesthesiology 90 (2): 484–491. February 1999. doi:10.1097/00000542-199902000-00024. PMID 9952156.
- ↑ "General anesthetics potentiate gamma-aminobutyric acid actions on gamma-aminobutyric acidA receptors expressed by Xenopus oocytes: lack of involvement of intracellular calcium". The Journal of Pharmacology and Experimental Therapeutics 263 (2): 569–578. November 1992. PMID 1331405.
- ↑ "The actions of ether, alcohol and alkane general anaesthetics on GABAA and glycine receptors and the effects of TM2 and TM3 mutations". British Journal of Pharmacology 129 (4): 731–743. February 2000. doi:10.1038/sj.bjp.0703087. PMID 10683198.
- ↑ "Effects of isoflurane and enflurane on GABAA and glycine receptors contribute equally to depressant actions on spinal ventral horn neurones in rats". British Journal of Anaesthesia 97 (5): 687–694. November 2006. doi:10.1093/bja/ael239. PMID 16973644.
- ↑ "Effects of halothane and isoflurane on calcium and potassium channel currents in canine coronary arterial cells". Anesthesiology 76 (6): 990–998. June 1992. doi:10.1097/00000542-199206000-00020. PMID 1318010.
- ↑ "Halothane, isoflurane, xenon, and nitrous oxide inhibit calcium ATPase pump activity in rat brain synaptic plasma membranes". Anesthesiology 82 (1): 108–117. January 1995. doi:10.1097/00000542-199501000-00015. PMID 7832292.
- ↑ "Protein kinases A and C phosphorylate......". http://www.diagnosticpathology.eu/content/ejp/ejp_001/ejp-001/001-14.htm.
- ↑ "Effect of anaesthesia and cardiopulmonary bypass on blood endocannabinoid concentrations during cardiac surgery". British Journal of Anaesthesia 105 (2): 139–144. August 2010. doi:10.1093/bja/aeq117. PMID 20525978.
- ↑ 23.0 23.1 "Use of anesthetic agents in neonates and young children". Anesthesia and Analgesia 104 (3): 509–520. March 2007. doi:10.1213/01.ane.0000255729.96438.b0. PMID 17312200. http://www.anesthesia-analgesia.org/cgi/content/full/104/3/509.
- ↑ "Isoflurane and desflurane at clinically relevant concentrations induce amyloid b-peptide oligomerization: An NMR study". Biochemical and Biophysical Research Communications 379 (3): 716–720. April 2009. doi:10.1016/j.bbrc.2008.12.092. PMID 19116131.
- ↑ "Anesthesia-Alzheimer disease link probed". JAMA 297 (16): 1760. April 2007. doi:10.1001/jama.297.16.1760. PMID 17456811.
- ↑ 26.0 26.1 26.2 26.3 26.4 26.5 "Isoflurane" (in en). PubChem. U.S. National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/3763.
- ↑ "The solubility of volatile anaesthetics in water at 25.0 degrees C using 19F NMR spectroscopy". Journal of Pharmaceutical and Biomedical Analysis 10 (1): 1–7. January 1992. doi:10.1016/0731-7085(92)80003-6. PMID 1391078.
- ↑ "Stereoselectivity of isoflurane in adhesion molecule leukocyte function-associated antigen-1". PLOS ONE 9 (5): e96649. 2014-05-06. doi:10.1371/journal.pone.0096649. PMID 24801074. Bibcode: 2014PLoSO...996649B.
- ↑ "The invention and development of enflurane, isoflurane, sevoflurane, and desflurane". Anesthesiology 108 (3): 531–533. March 2008. doi:10.1097/ALN.0b013e31816499cc. PMID 18292690.
- ↑ "Isomerism and anaesthetic drugs". Acta Anaesthesiologica Scandinavica. Supplementum 106: 83–90. August 1995. doi:10.1111/j.1399-6576.1995.tb04316.x. PMID 8533553.
- ↑ "Modern inhalation anesthetics: Potent greenhouse gases in the global atmosphere". Geophysical Research Letters 42 (5): 1606–1611. 2015. doi:10.1002/2014GL062785. Bibcode: 2015GeoRL..42.1606V. https://www.dora.lib4ri.ch/empa/islandora/object/empa%3A6687.
- ↑ "Advantages and guidelines for using isoflurane". The Veterinary Clinics of North America. Small Animal Practice 22 (2): 328–331. March 1992. doi:10.1016/s0195-5616(92)50626-x. PMID 1585568.
- ↑ "Isoflurane-Vet 100% w/w Inhalation vapour, liquid" (in en). https://www.noahcompendium.co.uk/.
External links
- "Isoflurane". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/isoflurane.
- U.S. Patent 3,535,388 - 1-chloro-2,2,2-trifluoroethyl difluoromethyl ether
- U.S. Patent 3,535,425 - 1-chloro-2,2,2-trifluoroethyl difluoromethyl ether as an anesthetic agent
 |

