(diff) ← Older revision | Latest revision (diff) | Newer revision → (diff)
Short description: Chemical compound
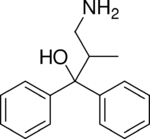
2-MDP (U-23807A) is a dissociative anaesthetic drug which has been found to be an NMDA antagonist and produces similar effects to PCP in animals. The levo or (−) isomer is the active form of the drug.[1][2] It also has stimulant effects, having only around one third the potency of amphetamine by weight, but with a long duration of action, lasting more than 24 hours from a single oral dose.[3]
Effects
The therapeutic action is said to exhibit appetite suppressant and antidepressant[4] like activity.
Synthesis
Error creating thumbnail: Unable to save thumbnail to destination
In a variation of the nitrile-Aldol reaction (also demonstrated for venlafaxine), combination of benzophenone (1) and propionitrile (2), in the presence of sodamide base and ethyl ether solvent, leads to 3-hydroxy-2-methyl-3,3-diphenylpropanenitrile [6275-86-1] (3). The reduction of the intermediate nitrile group with lithium aluminium hydride completed the synthesis of U-23,807A (4).
References
- ↑ "Phencyclidine-like behavioral effects of 2-methyl-3,3-diphenyl-3-propanolamine (2-MDP)". Pharmacology, Biochemistry, and Behavior 20 (2): 209–213. February 1984. doi:10.1016/0091-3057(84)90244-2. PMID 6718449.
- ↑ "2-Methyl-3,3-diphenyl-3-propanolamine (2-MDP) selectively antagonises N-methyl-aspartate (NMA)". Pharmacology, Biochemistry, and Behavior 24 (1): 23–25. January 1986. doi:10.1016/0091-3057(86)90038-9. PMID 3511477.
- ↑ "Antidepressants, Stimulants, Hallucinogens.". Annual Reports in Medicinal Chemistry (Academic Press) 2: 11–23, 18. January 1967. doi:10.1016/S0065-7743(08)61499-2.
- ↑ "A controlled evaluation of U-23,807A in the neurotic depressive syndrome". Current Therapeutic Research, Clinical and Experimental 9 (10): 514–516. October 1967. PMID 4964946.
- ↑ "Central nervous system agents. 2. Synthesis of diphenyl primary and secondary aminopropanols". Journal of Medicinal Chemistry 14 (11): 1100–1106. November 1971. doi:10.1021/jm00293a019. PMID 5115211.
|
|---|
Psychedelics
(5-HT2A
agonists) | | Benzofurans | |
|---|
Lyserg‐
amides | |
|---|
Phenethyl‐
amines | | 2C-x | | 25x-NBx | | 25x-NB3OMe | |
|---|
| 25x-NB4OMe | |
|---|
| 25x-NBF | |
|---|
| 25x-NBMD | |
|---|
| 25x-NBOH | |
|---|
| 25x-NBOMe | |
|---|
| Atypical structures | |
|---|
|
|---|
|
|---|
| 3C-x | |
|---|
| 4C-x | |
|---|
| DOx | |
|---|
| HOT-x | |
|---|
| MDxx | |
|---|
| Mescaline (subst.) | |
|---|
| TMAs |
- TMA
- TMA-2
- TMA-3
- TMA-4
- TMA-5
- TMA-6
|
|---|
| Others | |
|---|
|
|---|
| Piperazines | |
|---|
| Tryptamines | | alpha-alkyltryptamines | |
|---|
| x-DALT | |
|---|
| x-DET | |
|---|
| x-DiPT | |
|---|
| x-DMT |
- 4,5-DHP-DMT
- 2,N,N-TMT
- 4-AcO-DMT
- 4-HO-5-MeO-DMT
- 4,N,N-TMT
- 4-Propionyloxy-DMT
- 5,6-diBr-DMT
- 5-AcO-DMT
- 5-Bromo-DMT
- 5-MeO-2,N,N-TMT
- 5-MeO-4,N,N-TMT
- 5-MeO-α,N,N-TMT
- 5-MeO-DMT
- 5-N,N-TMT
- 7,N,N-TMT
- α,N,N-TMT
- (Bufotenin) 5-HO-DMT
- DMT
- Norbaeocystin
- (Psilocin) 4-HO-DMT
- (Psilocybin) 4-PO-DMT
|
|---|
| x-DPT | |
|---|
| Ibogaine-related | |
|---|
| x-MET | |
|---|
| x-MiPT | |
|---|
| Others | |
|---|
|
|---|
| Others | |
|---|
|
|---|
Dissociatives
(NMDAR
antagonists) | |
|---|
Deliriants
(mAChR
antagonists) | |
|---|
| Others | |
|---|
|
|---|
| Adamantanes | |
|---|
| Adenosine antagonists | |
|---|
| Alkylamines | |
|---|
| Ampakines | |
|---|
| Arylcyclohexylamines | |
|---|
| Benzazepines | |
|---|
| Cholinergics | |
|---|
| Convulsants | |
|---|
| Eugeroics | |
|---|
| Oxazolines | |
|---|
| Phenethylamines |
- 1-(4-Methylphenyl)-2-aminobutane
- 1-Methylamino-1-(3,4-methylenedioxyphenyl)propane
- 2-Fuoroamphetamine
- 2-Fuoromethamphetamine
- 2-OH-PEA
- 2-Phenyl-3-aminobutane
- 2,3-MDA
- 3-Fuoroamphetamine
- 3-Fluoroethamphetamine
- 3-Fluoromethcathinone
- 3-Methoxyamphetamine
- 3-Methylamphetamine
- 3,4-DMMC
- 4-BMC
- 4-CMC
- 4-Fluoroamphetamine
- 4-Fluoromethamphetamine
- 4-MA
- 4-Methylbuphedrone
- 4-Methylcathinone
- 4-MEAP
- 4-MMA
- 4-Methylpentedrone
- 4-MTA
- 6-FNE
- AL-1095
- Alfetamine
- a-Ethylphenethylamine
- Amfecloral
- Amfepentorex
- Amfepramone
- Amidephrine
- 2-Amino-1,2-dihydronaphthalene
- 2-Aminoindane
- 5-(2-Aminopropyl)indole
- 2-Aminotetralin
- Acridorex
- Amphetamine (Dextroamphetamine, Levoamphetamine)
- Amphetaminil
- Arbutamine
- β-Methylphenethylamine
- β-Phenylmethamphetamine
- Benfluorex
- Benzedrone
- Benzphetamine
- BDB
- BOH
- 3-Benzhydrylmorpholine
- BPAP
- Buphedrone
- Bupropion
- Butylone
- Camfetamine
- Cathine
- Cathinone
- Chlorphentermine
- Cilobamine
- Cinnamedrine
- Clenbuterol
- Clobenzorex
- Cloforex
- Clortermine
- Cypenamine
- D-Deprenyl
- Denopamine
- Dimethoxyamphetamine
- Dimethylamphetamine
- Dimethylcathinone
- Dobutamine
- DOPA (Dextrodopa, Levodopa)
- Dopamine
- Dopexamine
- Droxidopa
- EBDB
- Ephedrine
- Epinephrine
- Epinine
- Etafedrine
- Ethcathinone
- Ethylnorepinephrine
- Ethylone
- Etilamfetamine
- Etilefrine
- Famprofazone
- Fencamfamin
- Fencamine
- Fenethylline
- Fenfluramine (Dexfenfluramine, Levofenfluramine)
- Fenproporex
- Feprosidnine
- Flephedrone
- Fludorex
- Formetorex
- Furfenorex
- Gepefrine
- Hexapradol
- Hexedrone
- HMMA
- Hordenine
- 4-Hydroxyamphetamine
- 5-Iodo-2-aminoindane
- Ibopamine
- Indanylamphetamine
- Iofetamine
- Isoetarine
- Isoethcathinone
- Isoprenaline
- L-Deprenyl (Selegiline)
- Lefetamine
- Lisdexamfetamine
- Lophophine
- MBDB
- MDA (tenamfetamine)
- MDBU
- MDEA
- MDMA (midomafetamine)
- MDMPEA
- MDOH
- MDPR
- MDPEA
- Mefenorex
- Mephedrone
- Mephentermine
- Metanephrine
- Metaraminol
- Mesocarb
- Methamphetamine (Dextromethamphetamine, Levomethamphetamine)
- Methoxamine
- Methoxyphenamine
- MMA
- Methcathinone
- Methedrone
- Methoxyphenamine
- Methylenedioxycathinone
- Methylone
- Mexedrone
- MMDA
- MMDMA
- MMMA
- Morforex
- N,alpha-Diethylphenylethylamine
- N-Ethylbuphedrone
- N-Ethylhexedrone
- N,N-Dimethylphenethylamine
- Naphthylamphetamine
- Nisoxetine
- Norepinephrine
- Norfenefrine
- Norfenfluramine
- Normetanephrine
- L-Norpseudoephedrine
- Octopamine (drug)
- Orciprenaline
- Ortetamine
- Oxifentorex
- Oxilofrine
- PBA
- PCA
- PCMA
- PHA
- Pentorex
- Pentedrone
- Pentylone
- Phenatine
- Phenpromethamine
- Phentermine
- Phenylalanine
- Phenylephrine
- Phenylpropanolamine
- Pholedrine
- PIA
- PMA
- PMEA
- PMMA
- PPAP
- Phthalimidopropiophenone
- Prenylamine
- Propylamphetamine
- Pseudoephedrine
- Ropinirole
- Salbutamol (Levosalbutamol)
- Sibutramine
- Solriamfetol
- Synephrine
- Theodrenaline
- Tiflorex
- Tranylcypromine
- Tyramine
- Tyrosine
- Xylopropamine
- Zylofuramine
|
|---|
| Phenylmorpholines | |
|---|
| Piperazines | |
|---|
| Piperidines | |
|---|
| Pyrrolidines | |
|---|
| Racetams | |
|---|
| Tropanes | |
|---|
| Tryptamines | |
|---|
| Others | |
|---|
|
 | Original source: https://en.wikipedia.org/wiki/2-MDP. Read more |





 (0 votes)
(0 votes)