Chemistry:Chloroform
|
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Trichloromethane | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | R-20, TCM | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1888 | ||
| |||
| |||
| Properties | |||
| CHCl3 | |||
| Molar mass | 119.37 g·mol−1 | ||
| Appearance | Highly refractive colorless liquid | ||
| Odor | Sweet, minty, pleasant | ||
| Density | 1.564 g/cm3 (−20 °C) 1.489 g/cm3 (25 °C) 1.394 g/cm3 (60 °C) | ||
| Melting point | −63.5 °C (−82.3 °F; 209.7 K) | ||
| Boiling point | 61.15 °C (142.07 °F; 334.30 K) decomposes at 450 °C | ||
| 10.62 g/L (0 °C) 8.09 g/L (20 °C) 7.32 g/L (60 °C) | |||
| Solubility | Soluble in benzene Miscible in diethyl ether, oils, ligroin, alcohol, CCl4, CS2 | ||
| Solubility in acetone | ≥ 100 g/L (19 °C) | ||
| Solubility in dimethyl sulfoxide | ≥ 100 g/L (19 °C) | ||
| Vapor pressure | 0.62 kPa (−40 °C) 7.89 kPa (0 °C) 25.9 kPa (25 °C) 313 kPa (100 °C) 2.26 MPa (200 °C) | ||
Henry's law
constant (kH) |
3.67 L·atm/mol (24 °C) | ||
| Acidity (pKa) | 15.7 (20 °C) | ||
| UV-vis (λmax) | 250 nm, 260 nm, 280 nm | ||
| −59.30·10−6 cm3/mol | |||
| Thermal conductivity | 0.13 W/(m·K) (20 °C) | ||
Refractive index (nD)
|
1.4459 (20 °C) | ||
| Viscosity | 0.563 cP (20 °C) | ||
| Structure | |||
| Tetrahedral | |||
| 1.15 D | |||
| Thermochemistry | |||
Heat capacity (C)
|
114.25 J/(mol·K) | ||
Std molar
entropy (S |
202.9 J/(mol·K) | ||
Std enthalpy of
formation (ΔfH⦵298) |
−134.3 kJ/mol | ||
Gibbs free energy (ΔfG˚)
|
−71.1 kJ/mol | ||
Std enthalpy of
combustion (ΔcH⦵298) |
473.21 kJ/mol | ||
| Pharmacology | |||
| 1=ATC code }} | N01AB02 (WHO) | ||
| Hazards[9] | |||
| Main hazards | Decomposes to extremely toxic phosgene and hydrogen chloride in presence of light – IARC group 2B – Reproductive toxicity – Specific target organ toxicity (STOT)[4][5][6] | ||
| Safety data sheet | [1] | ||
| GHS pictograms |   
| ||
| GHS Signal word | Danger | ||
| H302, H315, H319, H331, H336, H351, H361d, H372 | |||
| P201, P202, P260, P264, P270, P271, P280, P281, P301+330+331, P310, P302+352, P304+340, P311, P305+351+338, P308+313, P314, P332+313, P337+313, P362, P403+233, P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | Nonflammable | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
704 mg/kg (mouse, dermal)[7] | ||
LC50 (median concentration)
|
9,617 ppm (rat, 4 hr)[8][clarification needed] | ||
LCLo (lowest published)
|
| ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
50 ppm (240 mg/m3)[5] | ||
REL (Recommended)
|
Ca ST 2 ppm (9.78 mg/m3) [60-minute][5] | ||
IDLH (Immediate danger)
|
500 ppm[5][clarification needed] | ||
| Related compounds | |||
Related compounds
|
| ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Chloroform, or trichloromethane (often abbreviated as TCM), is an organic compound with the formula CHCl
3 and a common solvent. It is a very volatile, colorless, strong-smelling, dense liquid produced on a large scale as a precursor to refrigerants and in turn PTFE.[10] Chloroform is a trihalomethane that serves as a powerful anesthetic, euphoriant, anxiolytic, and sedative when inhaled or ingested. Chloroform was used as an anesthetic between the 19th century and the first half of the 20th century.[11][12] It is miscible with many solvents but it is only very slightly soluble in water (only 8 g/L at 20°C).
Structure and name
The molecule adopts a tetrahedral molecular geometry with C3v symmetry.[13] The chloroform molecule can be viewed as a methane molecule with three hydrogen atoms replaced with three chlorine atoms, leaving a single hydrogen atom.
The name "chloroform" is a portmanteau of terchloride (tertiary chloride, a trichloride) and formyle, an obsolete name for the methylidene radical (CH) derived from formic acid.
Natural occurrence
The total global flux of chloroform through the environment is approximately 660000 tonnes per year,[14] and about 90% of emissions are natural in origin. Many kinds of seaweed produce chloroform, and fungi are believed to produce chloroform in soil.[15] Abiotic processes are also believed to contribute to natural chloroform productions in soils, although the mechanism is still unclear.[16]
As chloroform is a volatile organic compound,[17] it dissipates readily from soil and surface water and undergoes degradation in air to produce phosgene, dichloromethane, formyl chloride, carbon monoxide, carbon dioxide, and hydrogen chloride. Its half-life in air ranges from 55 to 620 days. Biodegradation in water and soil is slow. Chloroform does not significantly bioaccumulate in aquatic organisms.[18]
History
Chloroform was synthesized independently by several investigators c. 1831:
- Moldenhawer, a German pharmacist from Frankfurt an der Oder, appears to have produced chloroform in 1830 by mixing chlorinated lime with ethanol; however, he mistook it for Chloräther (chloric ether, 1,2-dichloroethane).[19][20]
- Samuel Guthrie, a U.S. physician from Sackets Harbor, New York, also appears to have produced chloroform in 1831 by reacting chlorinated lime with ethanol, and noted its anaesthetic properties; however, he also believed that he had prepared chloric ether.[21][22][23]
- Justus von Liebig carried out the alkaline cleavage of chloral. Liebig incorrectly states that the empirical formula of chloroform was C
2Cl
5 and named it "Chlorkohlenstoff" ("carbon chloride").[24][25] - Eugène Soubeiran obtained the compound by the action of chlorine bleach on both ethanol and acetone.[26]
In 1834, French chemist Jean-Baptiste Dumas determined chloroform's empirical formula and named it:[27] "Es scheint mir also erweisen, dass die von mir analysirte Substanz, … zur Formel hat: C2H2Cl6." (Thus it seems to me to show that the substance I analyzed … has as [its empirical] formula: C2H2Cl6.). [Note: The coefficients of his empirical formula should be halved.] ... "Diess hat mich veranlasst diese Substanz mit dem Namen 'Chloroform' zu belegen." (This had caused me to impose the name "chloroform" upon this substance [i.e., formyl chloride or chloride of formic acid].)
In 1835, Dumas prepared the substance by alkaline cleavage of trichloroacetic acid.
In 1842, Robert Mortimer Glover in London discovered the anaesthetic qualities of chloroform on laboratory animals.[28]
In 1847, Scottish obstetrician James Y. Simpson was the first to demonstrate the anaesthetic properties of chloroform in humans, provided by local pharmacist William Flockhart of Duncan, Flockhart and company,[29] and helped to popularize the drug for use in medicine.[30]
By the 1850s, chloroform was being produced on a commercial basis. In Britain, about 750,000 doses a week were being produced by 1895,[31] using the Liebig procedure, which retained its importance until the 1960s. Today, chloroform – along with dichloromethane – is prepared exclusively and on a massive scale by the chlorination of methane and chloromethane.[10]
Production
Industrially, chloroform is produced by heating a mixture of chlorine and either methyl chloride (CH
3Cl) or methane (CH
4).[10] At 400–500 °C, free radical halogenation occurs, converting these precursors to progressively more chlorinated compounds:
Chloroform undergoes further chlorination to yield carbon tetrachloride (CCl
4):
- CHCl
3 + Cl
2 → CCl
4 + HCl
The output of this process is a mixture of the four chloromethanes: chloromethane, methylene chloride (dichloromethane), trichloromethane (chloroform), and tetrachloromethane (carbon tetrachloride). These can then be separated by distillation.[10]
Chloroform may also be produced on a small scale via the haloform reaction between acetone and sodium hypochlorite:
Deuterochloroform
Deuterated chloroform is an isotopologue of chloroform with a single deuterium atom. CDCl
3 is a common solvent used in NMR spectroscopy. Deuterochloroform is produced by the reaction of hexachloroacetone with deuterium oxide.[32] The haloform process is now obsolete for production of ordinary chloroform. Deuterochloroform can also be prepared by reacting sodium deuteroxide with chloral hydrate.[33][34]
Inadvertent formation of chloroform
The haloform reaction can also occur inadvertently in domestic settings. Bleaching with hypochlorite generates halogenated compounds in side reactions; chloroform is the main byproduct.[35] Sodium hypochlorite solution (chlorine bleach) mixed with common household liquids such as acetone, methyl ethyl ketone, ethanol, or isopropyl alcohol can produce some chloroform, in addition to other compounds, such as chloroacetone or dichloroacetone.[citation needed]
Uses
In terms of scale, the most important reaction of chloroform is with hydrogen fluoride to give monochlorodifluoromethane (HCFC-22), a precursor in the production of polytetrafluoroethylene (Teflon) and other fluoropolymers:[10]
- CHCl
3 + 2 HF → CHClF
2 + 2 HCl
The reaction is conducted in the presence of a catalytic amount of mixed antimony halides. Chlorodifluoromethane is then converted to tetrafluoroethylene, the main precursor of Teflon.[36]
Solvent
The hydrogen attached to carbon in chloroform participates in hydrogen bonding,[37][38] making it a good solvent for many materials.
Worldwide, chloroform is also used in pesticide formulations, as a solvent for lipids, rubber, alkaloids, waxes, gutta-percha, and resins, as a cleansing agent, as a grain fumigant, in fire extinguishers, and in the rubber industry.[18][39] CDCl
3 is a common solvent used in NMR spectroscopy.[40]
Refrigerant
Trichloromethane is used as a precursor to make R-22 (chlorodifluoromethane). This is done by reacting it with a solution of Hydrofluoric acid (HF) which fluorinates the CHCl
3 molecule and releases hydrochloric acid as a byproduct.[41] Before the Montreal Protocol was enforced, most of the trichloromethane produced in the United States was used in the production of chlorodifluoromethane. However, its production remains high, as it is a key precursor of PTFE.[42]
Although trichloromethane has properties such as a low boiling point, and a low global warming potential of only 31 (compared to the 1760 of R-22), which gives it good refrigerating properties, there is little information to suggest that it has seen widespread use as a refrigerant in any consumer products.[43]
Lewis acid
In solvents such as CCl
4 and alkanes, chloroform hydrogen bonds to a variety of Lewis bases. HCCl
3 is classified as a hard acid, and the ECW model lists its acid parameters as EA = 1.56 and CA = 0.44.
Reagent
As a reagent, chloroform serves as a source of the dichlorocarbene intermediate CCl
2.[44] It reacts with aqueous sodium hydroxide, usually in the presence of a phase transfer catalyst, to produce dichlorocarbene, CCl
2.[45][46] This reagent effects ortho-formylation of activated aromatic rings, such as phenols, producing aryl aldehydes in a reaction known as the Reimer–Tiemann reaction. Alternatively, the carbene can be trapped by an alkene to form a cyclopropane derivative. In the Kharasch addition, chloroform forms the •CHCl
2 free radical in addition to alkenes.[citation needed]
Anaesthetic
The anaesthetic qualities of chloroform were first described in 1842 in a thesis by Robert Mortimer Glover, which won the Gold Medal of the Harveian Society for that year.[47][48] Glover also undertook practical experiments on dogs to prove his theories, refined his theories, and presented them in his doctoral thesis at the University of Edinburgh in the summer of 1847.
The Scottish obstetrician James Young Simpson was one of those required to read the thesis, but later claimed to have never read it and to have come to his own conclusions independently.[citation needed] On 4 November 1847, Simpson argued that he had discovered the anaesthetic qualities of chloroform in humans. He and two colleagues entertained themselves by trying the effects of various substances, and thus revealed the potential for chloroform in medical procedures.[29]
A few days later, during the course of a dental procedure in Edinburgh, Francis Brodie Imlach became the first person to use chloroform on a patient in a clinical context.[49]
In May 1848, Robert Halliday Gunning made a presentation to the Medico-Chirurgical Society of Edinburgh following a series of laboratory experiments on rabbits that confirmed Glover's findings and also refuted Simpson's claims of originality. The laboratory experiments that proved the dangers of chloroform were largely ignored.[50]
The use of chloroform during surgery expanded rapidly in Europe; for instance in the 1850s chloroform was used by the physician John Snow during the births of Queen Victoria's last two children.[51] In the United States, chloroform began to replace ether as an anesthetic at the beginning of the 20th century;[citation needed] it was abandoned in favor of ether on discovery of its toxicity, especially its tendency to cause fatal cardiac arrhythmias analogous to what is now termed "sudden sniffer's death". Some people used chloroform as a recreational drug or to attempt suicide.[52] One possible mechanism of action of chloroform is that it increases the movement of potassium ions through certain types of potassium channels in nerve cells.[53] Chloroform could also be mixed with other anaesthetic agents such as ether to make C.E. mixture, or ether and alcohol to make A.C.E. mixture.[citation needed]
In 1848, Hannah Greener, a 15-year-old girl who was having an infected toenail removed, died after being given the anaesthetic.[54] Her autopsy establishing the cause of death was undertaken by John Fife assisted by Robert Mortimer Glover.[28] A number of physically fit patients died after inhaling it. In 1848, however, John Snow developed an inhaler that regulated the dosage and so successfully reduced the number of deaths.[55]
The opponents and supporters of chloroform disagreed on the question of whether the medical complications were due to respiratory disturbance or whether chloroform had a specific effect on the heart. Between 1864 and 1910, numerous commissions in Britain studied chloroform but failed to come to any clear conclusions. It was only in 1911 that Levy proved in experiments with animals that chloroform can cause ventricular fibrillation.[citation needed] Despite this, between 1865 and 1920, chloroform was used in 80 to 95% of all narcoses performed in the UK and German-speaking countries. In Germany, comprehensive surveys of the fatality rate during anaesthesia were made by Gurlt between 1890 and 1897.[citation needed] At the same time in the UK the medical journal The Lancet carried out a questionnaire survey[56] and compiled a report detailing numerous adverse reactions to anesthetics, including chloroform.[57] In 1934, Killian gathered all the statistics compiled until then and found that the chances of suffering fatal complications under ether were between 1:14,000 and 1:28,000, whereas with chloroform the chances were between 1:3,000 and 1:6,000.[citation needed] The rise of gas anaesthesia using nitrous oxide, improved equipment for administering anesthetics, and the discovery of hexobarbital in 1932 led to the gradual decline of chloroform narcosis.[58]
The latest reported anaesthetic use of chloroform in the Western world dates to 1987, when the last doctor who used it retired, about 140 years after its first use.[59]
Criminal use
Chloroform has been used by criminals to knock out, daze, or even murder victims. Joseph Harris was charged in 1894 with using chloroform to rob people.[60] Serial killer H. H. Holmes used chloroform overdoses to kill his female victims. In September 1900, chloroform was implicated in the murder of the U.S. businessman William Marsh Rice. Chloroform was deemed a factor in the alleged murder of a woman in 1991, when she was asphyxiated while asleep.[61] In 2002, 13-year-old Kacie Woody was sedated with chloroform when she was abducted by David Fuller and during the time that he had her, before he shot and killed her.[62] In a 2007 plea bargain, a man confessed to using stun guns and chloroform to sexually assault minors.[63]
The use of chloroform as an incapacitating agent has become widely recognized, bordering on cliché, through the adoption by crime fiction authors of plots involving criminals' use of chloroform-soaked rags to render victims unconscious. However, it is nearly impossible to incapacitate someone using chloroform in this way.[64] It takes at least five minutes of inhalation of chloroform to render a person unconscious. Most criminal cases involving chloroform involve co-administration of another drug, such as alcohol or diazepam, or the victim being complicit in its administration. After a person has lost consciousness owing to chloroform inhalation, a continuous volume must be administered, and the chin must be supported to keep the tongue from obstructing the airway, a difficult procedure, typically requiring the skills of an anesthesiologist. In 1865, as a direct result of the criminal reputation chloroform had gained, the medical journal The Lancet offered a "permanent scientific reputation" to anyone who could demonstrate "instantaneous insensibility", i.e. loss of consciousness, using chloroform.[65]
Safety
Exposure
Chloroform is formed as a by-product of water chlorination, along with a range of other disinfection by-products, and it is therefore often present in municipal tap water and swimming pools. Reported ranges vary considerably, but are generally below the current health standard for total trihalomethanes (THMs) of 100 μg/L.[66] However, when considered in combination with other trihalomethanes often present in drinking water, the concentration of THMs often exceeds the recommended limit of exposure.[67]
While few studies have assessed the risks posed by chloroform exposure through drinking water in isolation from other THMs, many studies have shown that exposure to the general category of THMs, including chloroform, is associated with an increased risk of cancer of the bladder or lower GI tract.[68]
Historically, chloroform exposure may well have been higher, owing to its common use as an anesthetic, as an ingredient in cough syrups, and as a constituent of tobacco smoke, where DDT had previously been used as a fumigant.[69]
Pharmacology
Chloroform is well absorbed, metabolized, and eliminated rapidly by mammals after oral, inhalation, or dermal exposure. Accidental splashing into the eyes has caused irritation.[18] Prolonged dermal exposure can result in the development of sores as a result of defatting. Elimination is primarily through the lungs as chloroform and carbon dioxide; less than 1% is excreted in the urine.[39]
Chloroform is metabolized in the liver by the cytochrome P-450 enzymes, by oxidation to chloromethanol and by reduction to the dichloromethyl free radical. Other metabolites of chloroform include hydrochloric acid and diglutathionyl dithiocarbonate, with carbon dioxide as the predominant end-product of metabolism.[70]
Like most other general anesthetics and sedative-hypnotic drugs, chloroform is a positive allosteric modulator at GABAA receptors.[71] Chloroform causes depression of the central nervous system (CNS), ultimately producing deep coma and respiratory center depression.[70] When ingested, chloroform causes symptoms similar to those seen after inhalation. Serious illness has followed ingestion of 7.5 g (0.26 oz). The mean lethal oral dose in an adult is estimated at 45 g (1.6 oz).[18]
The anesthetic use of chloroform has been discontinued, because it caused deaths from respiratory failure and cardiac arrhythmias. Following chloroform-induced anesthesia, some patients suffered nausea, vomiting, hyperthermia, jaundice, and coma owing to hepatic dysfunction. At autopsy, liver necrosis and degeneration have been observed.[18]
Chloroform has induced liver tumors in mice and kidney tumors in mice and rats.[18] The hepatotoxicity and nephrotoxicity of chloroform is thought to be due largely to phosgene, one of its metabolites.[70]
Conversion to phosgene
Chloroform converts slowly in the presence of UV light and air to the extremely poisonous gas, phosgene (COCl
2), releasing HCl in the process.[72]
- 2 CHCl
3 + O
2 → 2 COCl
2 + 2 HCl
To prevent accidents, commercial chloroform is stabilized with ethanol or amylene, but samples that have been recovered or dried no longer contain any stabilizer. Amylene has been found to be ineffective, and the phosgene can affect analytes in samples, lipids, and nucleic acids dissolved in or extracted with chloroform.[73] Phosgene and HCl can be removed from chloroform by washing with saturated aqueous carbonate solutions, such as sodium bicarbonate. This procedure is simple and results in harmless products. Phosgene reacts with water to form carbon dioxide and HCl,[74] and the carbonate salt neutralizes the resulting acid.[75]
Suspected samples can be tested for phosgene using filter paper (treated with 5% diphenylamine, 5% dimethylaminobenzaldehyde in ethanol, and then dried), which turns yellow in phosgene vapour. There are several colorimetric and fluorometric reagents for phosgene, and it can also be quantified using mass spectrometry.[76]
Regulation
Chloroform is suspected of causing cancer (i.e. it is possibly carcinogenic, IARC Group 2B) as per the International Agency for Research on Cancer (IARC) Monographs.[77]
It is classified as an extremely hazardous substance in the United States, as defined in Section 302 of the US Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities that produce, store, or use it in significant quantities.[78]
Bioremediation of chloroform
Some anaerobic bacteria use chloroform for respiration, termed organohalide respiration, converting it to dichloromethane.[79][80]
Gallery
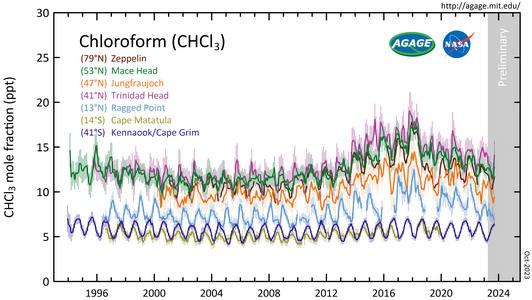
CHCl
3 measured by the Advanced Global Atmospheric Gases Experiment (AGAGE) in the lower atmosphere (troposphere) at stations around the world. Abundances are given as pollution free monthly mean mole fractions in parts-per-trillion (ppt).
References
- ↑ "Front Matter". Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 661. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4. "The retained names 'bromoform' for HCBr3, 'chloroform' for HCCl3, and 'iodoform' for HCI3 are acceptable in general nomenclature. Preferred IUPAC names are substitutive names."
- ↑ Gregory, William, A Handbook of Organic Chemistry (Third edition corrected and much extended), 1852, page 177
- ↑ Daniel Pereira Gardner, Medicinal Chemistry for the Use of Students and the Profession: Being a Manual of the Science, with Its Applications to Toxicology, Physiology, Therapeutics, Hygiene, Etc (1848), page 271
- ↑ "Part 3 Health Hazards". Globally Harmonized System of Classification and Labelling of Chemicals (GHS). United Nations. http://www.unece.org/fileadmin/DAM/trans/danger/publi/ghs/ghs_rev02/English/03e_part3.pdf.
- ↑ 5.0 5.1 5.2 5.3 NIOSH Pocket Guide to Chemical Hazards. "#0127". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0127.html.
- ↑ Toxicity on PubChem
- ↑ Lewis, Richard J. (2012). Sax's Dangerous Properties of Industrial Materials (12th ed.). ISBN 978-0-470-62325-1.
- ↑ 8.0 8.1 "Chloroform". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/67663.html.
- ↑ "PubChem: Safety and Hazards – GHS Classification". National Center for Biotechnology Information, U.S. National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/6212#section=Safety-and-Hazards.
- ↑ 10.0 10.1 10.2 10.3 10.4 Rossberg, M.. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a06_233.pub2.
- ↑ "Ether and Chloroform". http://www.history.com/topics/ether-and-chloroform.
- ↑ "Chloroform [MAK Value Documentation, 2000]". The MAK-Collection for Occupational Health and Safety. 2012. pp. 20–58. doi:10.1002/3527600418.mb6766e0014. ISBN 978-3-527-60041-0.
- ↑ "Illustrated Glossary of Organic Chemistry - Chloroform". http://www.chem.ucla.edu/~harding/IGOC/C/chloroform.html.
- ↑ Gribble, Gordon W. (2004). "Natural Organohalogens: A New Frontier for Medicinal Agents?". Journal of Chemical Education 81 (10): 1441. doi:10.1021/ed081p1441. Bibcode: 2004JChEd..81.1441G.
- ↑ Cappelletti, M. (2012). "Microbial degradation of chloroform". Applied Microbiology and Biotechnology 96 (6): 1395–409. doi:10.1007/s00253-012-4494-1. PMID 23093177.
- ↑ Jiao, Yi (2018). "Halocarbon Emissions from a Degraded Forested Wetland in Coastal South Carolina Impacted by Sea Level Rise". ACS Earth and Space Chemistry 2 (10): 955–967. doi:10.1021/acsearthspacechem.8b00044. Bibcode: 2018ESC.....2..955J.
- ↑ "Complete list of VOC's". http://aqt-vru.com/emissions/complete-list-of-vocs/.
- ↑ 18.0 18.1 18.2 18.3 18.4 18.5 Chloroform, CICAD, 58, World Health Organization, 2004, https://www.who.int/ipcs/publications/cicad/en/cicad58.pdf
- ↑ Moldenhawer (1830). "Verfahren den Spiritus von dem Fuselöl auf leichte Weise zu befreien". Magazin für Pharmacie 8 (31): 222–227. https://books.google.com/books?id=a_E3AAAAMAAJ&pg=PA222. Retrieved 6 May 2016.
- ↑ Defalque, Ray J.; Wright, A. J. (2000). "Was chloroform produced before 1831?". Anesthesiology 92 (1): 290–291. doi:10.1097/00000542-200001000-00060. PMID 10638939.
- ↑ Guthrie, Samuel (1832). "New mode of preparing a spirituous solution of chloric ether". The American Journal of Science and Arts 21: 64–65 and 405–408. https://books.google.com/books?id=iuzRAAAAMAAJ&pg=PA64. Retrieved 6 May 2016.
- ↑ Guthrie, Ossian (1887). Memoirs of Dr. Samuel Guthrie, and the History of the Discovery of Chloroform. Chicago: George K. Hazlitt & Co.. p. 1. https://archive.org/details/39002011125375.med.yale.edu.
- ↑ Stratmann, Linda (2003). "Chapter 2". Chloroform: The Quest for Oblivion. Stroud: Sutton Publishing. ISBN 978-0-7524-9931-4. https://books.google.com/books?id=VvA7AwAAQBAJ&pg=PT30. Retrieved 6 May 2016.
- ↑ Liebig, Justus von (1831). "Ueber die Zersetzung des Alkohols durch Chlor". Annalen der Physik und Chemie 99 (11): 444. doi:10.1002/andp.18310991111. Bibcode: 1831AnP....99..444L. https://babel.hathitrust.org/cgi/pt?id=uc1.a0002753747;view=1up;seq=462. Retrieved 6 May 2016.
- ↑ Liebig, Justus von (1832). "Ueber die Verbindungen, welche durch die Einwirkung des Chlors auf Alkohol, Aether, ölbildendes Gas und Essiggeist entstehen". Annalen der Physik und Chemie 100 (2): 243–295. doi:10.1002/andp.18321000206. Bibcode: 1832AnP...100..243L. https://babel.hathitrust.org/cgi/pt?id=wu.89048351662&view=1up&seq=861.
On pages 259–265, Liebig describes Chlorkohlenstoff ("carbon chloride", chloroform), but on p. 264, Liebig incorrectly states that the empirical formula of chloroform is C2Cl5. - ↑ Soubeiran, Eugène (1831). "Recherches sur quelques combinaisons du chlore". Annales de Chimie et de Physique. Série 2 48: 113–157. https://babel.hathitrust.org/cgi/pt?id=ien.35556014127963;view=1up;seq=115. Retrieved 6 May 2016.
- Reprinted in Soubeiran, Eugène (1831). "Recherches sur quelques combinaisons du chlore". Journal de Pharmacie et des Sciences Accessoires 17: 657–672. https://books.google.com/books?id=QP1BAAAAcAAJ&pg=PA657. Retrieved 6 May 2016.
- Reprinted in Soubeiran, Eugène (1832). "Suite des recherches sur quelques combinaisons du chlore". Journal de Pharmacie et des Sciences Accessoires 18: 1–24. https://books.google.com/books?id=aBZJAQAAMAAJ&pg=PA1. Retrieved 6 May 2016.
- ↑ Dumas, J.-B. (1834). "Récherches rélative à l'action du chlore sur l'alcool". L'Institut, Journal Général des Sociétés et Travaux Scientifiques de la France et de l'Étranger 2: 106–108 and 112–115.
- Reprinted in Dumas, J.-B. (1834). "Untersuchung über die Wirkung des Chlors auf den Alkohol". Annalen der Physik und Chemie 107 (42): 657–673. doi:10.1002/andp.18341074202. Bibcode: 1834AnP...107..657D. https://babel.hathitrust.org/cgi/pt?id=umn.31951d00316736l;view=1up;seq=668. Retrieved 12 May 2016.
On p. 653, Dumas states chloroform's empirical formula:
- "Es scheint mir also erweisen, dass die von mir analysirte Substance, … zur Formel hat: C
2H
2Cl
6." (Thus it seems to me to show that the substance [that was] analyzed by me … has as [its empirical] formula: C
2H
2Cl
6.) [Note: The coefficients of his empirical formula must be halved.]
- "Es scheint mir also erweisen, dass die von mir analysirte Substance, … zur Formel hat: C
- Dumas then notes that chloroform's simple empirical formula resembles that of formic acid. Furthermore, if chloroform is boiled with potassium hydroxide, one of the products is potassium formate. On p. 654, Dumas names chloroform:
- "Diess hat mich veranlasst diese Substanz mit dem Namen 'Chloroform' zu belegen." (This caused me to bestow this substance with the name "chloroform" [i.e., formyl chloride or chloride of formic acid].)
- Reprinted in Dumas, J.-B. (1835). "Ueber die Wirkung des Chlors auf den Alkohol". Annalen der Pharmacie 16 (2): 164–171. doi:10.1002/jlac.18350160213. https://babel.hathitrust.org/cgi/pt?id=uva.x002457902;view=1up;seq=542. Retrieved 12 May 2016.
- Reprinted in Dumas, J.-B. (1834). "Untersuchung über die Wirkung des Chlors auf den Alkohol". Annalen der Physik und Chemie 107 (42): 657–673. doi:10.1002/andp.18341074202. Bibcode: 1834AnP...107..657D. https://babel.hathitrust.org/cgi/pt?id=umn.31951d00316736l;view=1up;seq=668. Retrieved 12 May 2016.
- ↑ 28.0 28.1 Defalque, R. J.; Wright, A. J. (2004). "The short, tragic life of Robert M. Glover". Anaesthesia 59 (4): 394–400. doi:10.1111/j.1365-2044.2004.03671.x. PMID 15023112. http://www.ph.ucla.edu/epi/snow/anaesthesia59_394_400_2004.pdf.
- ↑ 29.0 29.1 Gordon, H. Laing (November 2002). Sir James Young Simpson and Chloroform (1811–1870). Minerva Group. pp. 106–109. ISBN 978-1-4102-0291-8. https://books.google.com/books?id=pYer05UwKBYC&pg=PA106. Retrieved 5 January 2016.
- ↑ "Sir James Young Simpson". Encyclopædia Britannica. https://www.britannica.com/EBchecked/topic/545447/Sir-James-Young-Simpson-1st-Baronet. Retrieved 23 August 2013.
- ↑ Worling, P.M. (1998). "Duncan and Flockhart: the Story of Two Men and a Pharmacy". Pharmaceutical Historian 28 (2): 28–33. PMID 11620310.
- ↑ Paulsen, P. J.; Cooke, W. D. (1 September 1963). "Preparation of Deuterated Solvents for Nuclear Magnetic Resonance Spectrometry.". Analytical Chemistry 35 (10): 1560. doi:10.1021/ac60203a072.
- ↑ Breuer, F. W. (1935). "Chloroform-d (Deuteriochloroform)1". Journal of the American Chemical Society 57 (11): 2236–2237. doi:10.1021/ja01314a058.
- ↑ Kluger, Ronald (1964). "A Convenient Preparation of Chloroform-d1". The Journal of Organic Chemistry 29 (7): 2045–2046. doi:10.1021/jo01030a526.
- ↑ Süss, Hans Ulrich. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH.
- ↑ "Chlorodifluoromethane | chemical compound" (in en). https://www.britannica.com/science/chlorodifluoromethane.
- ↑ Wiley, G. R.; Miller, S. I. (1972). "Thermodynamic parameters for hydrogen bonding of chloroform with Lewis bases in cyclohexane. Proton magnetic resonance study". Journal of the American Chemical Society 94 (10): 3287–3293. doi:10.1021/ja00765a001.
- ↑ Kwak, K.; Rosenfeld, D. E.; Chung, J. K.; Fayer, M. D. (2008). "Solute-solvent complex switching dynamics of chloroform between acetone and dimethylsulfoxide-two-dimensional IR chemical exchange spectroscopy". The Journal of Physical Chemistry B 112 (44): 13906–13915. doi:10.1021/jp806035w. PMID 18855462.
- ↑ 39.0 39.1 Leikin, Jerrold B.; Paloucek, Frank P., eds (2008). "Chloroform". Poisoning and Toxicology Handbook (4th ed.). Informa. p. 774.
- ↑ Fulmer, Gregory R.; Miller, Alexander J. M.; Sherden, Nathaniel H.; Gottlieb, Hugo E.; Nudelman, Abraham; Stoltz, Brian M.; Bercaw, John E.; Goldberg, Karen I. (2010). "NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist". Organometallics 29 (9): 2176–2179. doi:10.1021/om100106e. https://authors.library.caltech.edu/18475/2/om100106e_si_001.pdf.
- ↑ "Chloroform (CHEBI:35255)". https://www.ebi.ac.uk/chebi/es/searchId.do?printerFriendlyView=true&locale=null&chebiId=35255&viewTermLineage=null&structureView=&.
- ↑ "Production, import/export, use, and disposal". https://www.atsdr.cdc.gov/ToxProfiles/tp6-c4.pdf.
- ↑ "Chloroform as a pollutant". The Encyclopedia of World Problems. http://encyclopedia.uia.org/en/problem/chloroform-pollutant#:~:text=Most%20chloroform%20is%20manufactured%20to,spot%20removers%2C%20and%20various%20solvents.
- ↑ Srebnik, M.; Laloë, E. (2001). Encyclopedia of Reagents for Organic Synthesis. Wiley. doi:10.1002/047084289X.rc105. ISBN 978-0-471-93623-7.
- ↑ Vogel, E.; Klug, W.; Breuer, A. (1988). "1,6-Methano[10annulene"]. Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv6p0731.; Collective Volume, 6, pp. 731
- ↑ Gokel, G. W.; Widera, R. P.; Weber, W. P. (1988). "Phase-Transfer Hofmann Carbylamine Reaction: tert-Butyl Isocyanide". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv6p0232.; Collective Volume, 6, pp. 232
- ↑ Perkins-McVey, Matthew (10 November 2023). "“A new order of poisonous substances”: revisiting Robert M. Glover’s dissertation on the physiological effects of bromine, chlorine, and iodine compounds". Naunyn-Schmiedeberg's Archives of Pharmacology: 1. doi:10.1007/s00210-023-02820-y. https://www.researchgate.net/publication/375556103_A_new_order_of_poisonous_substances_revisiting_Robert_M_Glover's_dissertation_on_the_physiological_effects_of_bromine_chlorine_and_iodine_compounds. Retrieved 27 January 2024.
- ↑ Glover, Robert M. (1 October 1842). "On the Physiological and Medicinal Properties of Bromine and Its Compounds; Also on the Analogies between the Physiological and Medicinal Properties of These Bodies, and Those of Chlorine and Iodine, with Their Correspondent Compounds; Being the Harveian Prize Essay for 1842.". Edinburgh medical and surgical journal 58 (153): 335-364. PMID 30330609. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5789197/. Retrieved 27 January 2024.
- ↑ Dingwall (April 2004). "A pioneering history: dentistry and the Royal College of Surgeons of Edinburgh". http://historyofdentistry.co.uk/index_htm_files/2004Apr2.pdf.
- ↑ Baillie, T. W. (2003). "Robert Halliday Gunning and the Victoria Jubilee Prizes". Scottish Medical Journal 48 (2): 54–57. doi:10.1177/003693300304800209. PMID 12774598. https://www.royalsoced.org.uk/cms/files/research_awards/prizes/prize_lists/gunning_victoria_history.pdf. Retrieved 2016-08-18.
- ↑ "Anesthesia and Queen Victoria". http://www.ph.ucla.edu/epi/snow/victoria.html.
- ↑ Martin, William (3 July 1886). "A Case of Chloroform Poisoning; Recovery". British Medical Journal 2 (1331): 16–17. doi:10.1136/bmj.2.1331.16-a. PMID 20751619.
- ↑ Patel, Amanda J.; Honoré, Eric; Lesage, Florian; Fink, Michel; Romey, Georges; Lazdunski, Michel (May 1999). "Inhalational anesthetics activate two-pore-domain background K+ channels". Nature Neuroscience 2 (5): 422–426. doi:10.1038/8084. PMID 10321245.
- ↑ Knight, Paul R. III; Bacon, Douglas R. (2002). "An Unexplained Death: Hannah Greener and Chloroform". Anesthesiology 96 (5): 1250–1253. doi:10.1097/00000542-200205000-00030. PMID 11981167.
- ↑ Snow, John (1858). On Chloroform and Other Anaesthetics and Their Action and Administration. London : John Churchill. pp. 82–85. https://archive.org/stream/onchloroformothe1858snow#page/82/mode/2up/search/inhaler.
- ↑ Anonymous. (1890). "The Lancet Inquiry into the Mortality Under Anaesthetics.". Lancet 145 (3472): 612–13.
- ↑ Anonymous. (1893). "Report of The Lancet Commission appointed to investigate the subject of the administration of chloroform and other anesthetics from a clinical standpoint.". Lancet 141 (3629): 629–38.
- ↑ Wawersik, J. (1997). "History of chloroform anesthesia". Anesthesiology and Reanimation 22 (6): 144–152. PMID 9487785.
- ↑ Stratmann, Linda (2003). Chloroform: The Quest for Oblivion. Stroud: Sutton Publishing. ISBN 978-0-7524-9931-4.
- ↑ "Knock-out and Chloroform". The Philadelphia Record. 9 February 1894. https://news.google.com/newspapers?id=Ec1VAAAAIBAJ&pg=2904,2720400&dq=chloroform+knockout&hl=en.
- ↑ "Chloroform case retrial underway". Record-Journal. 7 July 1993. https://news.google.com/newspapers?id=I91HAAAAIBAJ&pg=2367,1007950&dq=chloroform+knockout&hl=en.
- ↑ Cathy Frye - Arkansas Democrat-Gazette (2003-12-17). "But not forgotten". https://www.arkansasonline.com/news/2003/dec/17/not-forgotten/.
- ↑ "Man admits to raping friends' daughters". USA Today. 6 November 2007. https://www.usatoday.com/news/nation/2007-11-06-chloroform-rapes_N.htm.
- ↑ Payne, J. P. (July 1998). "The criminal use of chloroform". Anaesthesia 53 (7): 685–690. doi:10.1046/j.1365-2044.1998.528-az0572.x. PMID 9771177.
- ↑ "Medical Annotation: Chloroform amongst Thieves". The Lancet 2 (2200): 490–491. 1865. doi:10.1016/s0140-6736(02)58434-8.
- ↑ Nieuwenhuijsen, MJ; Toledano, MB; Elliott, P (8 August 2000). "Uptake of chlorination disinfection by-products; a review and a discussion of its implications for exposure assessment in epidemiological studies.". Journal of Exposure Analysis and Environmental Epidemiology 10 (6 Pt 1): 586–99. doi:10.1038/sj.jea.7500139. PMID 11140442.
- ↑ Group, Environmental Working. "EWG's Tap Water Database: Contaminants in Your Water" (in en). https://www.ewg.org/tapwater/reviewed-disinfection-byproducts.php.
- ↑ "Public Health Goals for Trihalomethanes in Drinking Water". p. 93. https://oehha.ca.gov/media/downloads/water/chemicals/phg/thmsphg020720.pdf.
- ↑ Yin-Tak Woo, David Y. Lai, Joseph C. Arcos Aliphatic and Polyhalogenated Carcinogens: Structural Bases and Biological
- ↑ 70.0 70.1 70.2 Fan, Anna M. (2005). "Chloroform". Encyclopedia of Toxicology. 1 (2nd ed.). Elsevier. pp. 561–565.
- ↑ Jenkins, Andrew; Greenblatt, Eric P.; Faulkner, Howard J.; Bertaccini, Edward; Light, Adam; Lin, Audrey; Andreasen, Alyson; Viner, Anna et al. (2001-03-15). "Evidence for a Common Binding Cavity for Three General Anesthetics within the GABAA Receptor" (in en). Journal of Neuroscience 21 (6): RC136. doi:10.1523/JNEUROSCI.21-06-j0002.2001. ISSN 0270-6474. PMID 11245705.
- ↑ "Chloroform and Phosgene, Chemical Hygiene and Safety". http://www.earlham.edu/chemical-hygiene-and-safety/safety-topics/chloroform-and-phosgene/.
- ↑ Turk, Eric (2 March 1998). "Phosgene from Chloroform". Chemical & Engineering News 76 (9): 6. doi:10.1021/cen-v076n009.p006. http://pubs.acs.org/cen/safety/19980302.html. Retrieved 13 August 2012.
- ↑ "phosgene (chemical compound)". https://www.britannica.com/EBchecked/topic/457363/phosgene.
- ↑ Manogue, W. H.; Pigford, R. L. (September 1960). "The kinetics of the absorption of phosgene into water and aqueous solutions" (in en). AIChE Journal 6 (3): 494–500. doi:10.1002/aic.690060329. ISSN 0001-1541. Bibcode: 1960AIChE...6..494M. https://onlinelibrary.wiley.com/doi/10.1002/aic.690060329.
- ↑ Cheng, Xueheng; Gao, Quanyin; Smith, Richard D.; Simanek, Eric E.; Mammen, Mathai; Whitesides, George M. (1996). "Characterization of Hydrogen-Bonded Aggregates in Chloroform by Electrospray Ionization Mass Spectrometry". The Journal of Organic Chemistry 61 (6): 2204–2206. doi:10.1021/jo951345g. ISSN 0022-3263. https://www.academia.edu/33298377.
- ↑ "Chloroform". https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono73-10.pdf.
- ↑ "40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities". Code of Federal Regulations (Government Printing Office). http://edocket.access.gpo.gov/cfr_2008/julqtr/pdf/40cfr355AppA.pdf. Retrieved 29 October 2011.
- ↑ Shuiquan Tang; Elizabeth A. Edwards (2013). "Identification of Dehalobacter reductive dehalogenases that catalyse dechlorination of chloroform, 1,1,1-trichloroethane and 1,1-dichloroethane". Philos Trans R Soc Lond B Biol Sci 368 (1616): 20120318. doi:10.1098/rstb.2012.0318. PMID 23479748.
- ↑ Jugder, Bat-Erdene; Ertan, Haluk; Wong, Yie Kuan; Braidy, Nady; Manefield, Michael; Marquis, Christopher P.; Lee, Matthew (2016-08-10). "Genomic, transcriptomic and proteomic analyses of Dehalobacter UNSWDHB in response to chloroform" (in en). Environmental Microbiology Reports 8 (5): 814–824. doi:10.1111/1758-2229.12444. ISSN 1758-2229. PMID 27452500. Bibcode: 2016EnvMR...8..814J.
External links
- Chloroform "The Molecular Lifesaver" – An article at Oxford University providing facts about chloroform.
- Chloroform Administration – a short film of anaesthetic chloroform application, filmed in the 1930s
- Concise International Chemical Assessment Document 58
- IARC Summaries & Evaluations: Vol. 1 (1972), Vol. 20 (1979), Suppl. 7 (1987), Vol. 73 (1999)
- International Chemical Safety Card 0027
- NIOSH Pocket Guide to Chemical Hazards. "#0127". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0127.html.
- NIST Standard Reference Database
 |