Chemistry:Prasterone enanthate
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | With estradiol valerate: Gynodian Depot, others |
| Other names | DHEA enanthate; Prasterone heptanoate; DHEA heptanoate; DHEA-E; EDHEA; SH-90300-D; SH-70833-D (with EV); Androst-5-en-3β-ol-17-one 3β-heptanoate |
| Routes of administration | Intramuscular injection |
| Drug class | Androgen; Anabolic steroid; Androgen ester; Estrogen; Neurosteroid |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | IM: 100%[1] |
| Metabolites | • Prasterone (DHEA)[1] • Others[1] |
| Elimination half-life | IM: 9 days[1] IV: 44 minutes[1] |
| Duration of action | 18 days[2] |
| Excretion | Urine, feces[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C26H40O3 |
| Molar mass | 400.603 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Prasterone enanthate, also known as dehydroepiandrosterone enanthate (DHEA-E) and sold in combination with estradiol valerate under the brand name Gynodian Depot among others, is a weak androgen, estrogen, and neurosteroid medication which is used as a component of menopausal hormone therapy to treat menopausal symptoms in women.[3][1][4][5][6][7][8][9][10] It is available only as an injectable preparation in combination with estradiol valerate.[3][11][12][13] The medication is given by injection into muscle typically once every 4 weeks.[3][1][4]
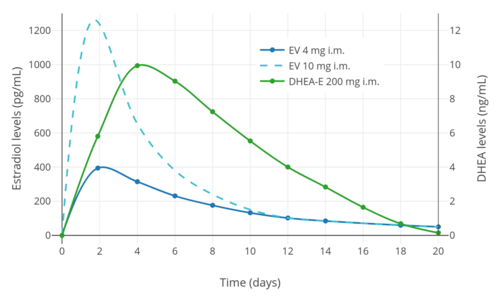
Prasterone enanthate is a synthetic androgen, estrogen, and neurosteroid.[3][1][4] It is a steroid ester and a long-lasting prodrug of prasterone (dehydroepiandrosterone; DHEA) in the body.[3][1][4] Prasterone is a naturally occurring prohormone of androgens and estrogens and hence is an agonist of the androgen and estrogen receptors, the respective biological targets of androgens like testosterone and estrogens like estradiol.[14][15] Prasterone also has a variety of activities of its own, including neurosteroid and other activities.[15] An injection of prasterone enanthate has a duration of action in terms of elevated prasterone levels of about 18 days.[3][1][4]
The combination of estradiol valerate and prasterone enanthate was developed as early as 1966 and was introduced for medical use in 1975.[16][17] The formulation is marketed widely throughout Europe, and is also available in several Latin American countries and in Egypt.[11][12][18][13][19] It is not available in any predominantly English-speaking countries.[11][19]
Medical uses
The combination of estradiol valerate and prasterone enanthate is used in menopausal hormone therapy to treat menopausal symptoms in peri- and postmenopausal women.[3][16] Estradiol valerate serves as an estrogen in the preparation, while prasterone enanthate is intended to serve as a weak androgen.[3][16] It is thought that the inclusion of prasterone enanthate in the formulation may provide additional psychotropic benefits.[16][20][21][22]
Available forms
Prasterone enanthate is available only as a combination formulation of 4 mg estradiol valerate and 200 mg prasterone enanthate in oil for depot intramuscular injection.[12][13][11]
Side effects
Prasterone enanthate, in combination with estradiol valerate at the dosages used clinically, has no masculinizing side effects.[16] This is in contrast to combinations of estrogens with other androgens, such as testosterone esters.[16]
The following is a list of possible side-effects that may occur in medicines that contain Estradiol Valerate / Prasterone Enanthate. This is not a comprehensive list. These side-effects are possible, but do not always occur. Some of the side-effects may be rare but serious. Consult your doctor if you observe any of the following side-effects, especially if they do not go away.
Dysmenorrhea Vaginitis Ovarian cancer Endometrial hyperplasia Endometrial cancer Breast cancer Stroke Increase in blood pressure Pulmonary embolism Nausea Vomiting Abdominal cramps Bloating Cholestatic jaundice Pruritus Rash Dizziness
Estradiol Valerate / Prasterone Enanthate may also cause side-effects not listed here.[23]
Pharmacology
Pharmacodynamics
Pharmacokinetics
The pharmacokinetics of prasterone enanthate have been assessed in a number of studies.[2][25]
Prasterone enanthate is a prodrug of prasterone in the body.[3][1][2] It is completely hydrolyzed into prasterone and heptanoic acid (enanthic acid) following absorption from the tissue depot after intramuscular injection.[1]
Levels of DHEA peak at about 9 ng/mL within 1 to 4 days of an injection of prasterone enanthate.[1] Subsequently, DHEA levels return to baseline by about 18 days following the injection.[1] Prasterone enanthate has an elimination half-life of about 9 days.[1] The plasma half-life of DHEA/prasterone enanthate following an intravenous injection is about 44 minutes.[1] The half-lives of DHEA metabolites range up to 3.6 days.[1]
Within 30 days, 91% of a dose of prasterone enanthate is eliminated.[1] Approximately 94% is excreted in urine and 6% in feces.[1] Prasterone enanthate is eliminated mainly in the form of metabolites and conjugates.[1]
Chemistry
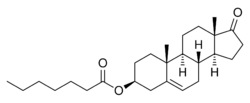
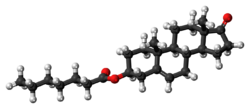
Prasterone enanthate, also known as 5-dehydroepiandrosterone 3β-enanthate or as androst-5-en-3β-ol-17-one 3β-heptanoate, is a synthetic androstane steroid and the C3β heptanoate (enanthate) ester of prasterone (5-dehydroepiandrosterone).[26][27][18]
History
Prasterone enanthate was patented by Schering in 1968 and 1971.[13][18] The combination of estradiol valerate and prasterone enanthate was developed and marketed by Schering, was first tested clinically as early as 1966, was first described in the scientific literature in 1972, and was first introduced for medical use in April 1975.[16][17][28][13]
Society and culture
Brand names
The major brand name of the combination of estradiol valerate and prasterone enanthate is Gynodian Depot.[11][12][13][19] Other brand names of this formulation include Binodian Depot, Cidodian Depot, Klimax, and Supligol NF.[11][12][13][19]
Availability
The combination of estradiol valerate and prasterone enanthate is marketed widely throughout Europe, and is also available in several Latin American countries and in Egypt.[11][12][18][13][19] In Europe, it is available in Austria, the Czech Republic, Germany , Italy, Poland , Russia , Spain , and Switzerland .[11][12][18][13][19] In Latin America, it is available in Argentina , Chile , Mexico, and Venezuela.[11][19] The medication is not available in any predominantly English-speaking countries, including the United States , Canada , the United Kingdom , Ireland, Australia , New Zealand, or South Africa .[11][19]
See also
- Estradiol valerate/prasterone enanthate
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 "Gynodian® Depot". Bayer (Schweiz) AG. compendium.ch. 16 October 2017. https://compendium.ch/FrmMainMonographie.aspx?Id=4a7e55ac-b11f-4d80-96d0-b468ab0eb4e3&lang=de&MonType=fi&start=1.
- ↑ 2.0 2.1 2.2 2.3 "Plasma levels of dehydroepiandrosterone and 17 beta-estradiol after intramuscular administration of Gynodian-Depot in 3 women". Hormone Research 17 (2): 84–89. 1983. doi:10.1159/000179680. PMID 6220949.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 (in de) Das Klimakterium – Pathophysiologie, Klinik, Therapie. Stuttgart, Germany: Thieme Verlag. 1987. p. 122. ISBN 978-3137008019.
- ↑ 4.0 4.1 4.2 4.3 4.4 "Modern Medicine". http://www.meppo.com/pdf/drugs/859-GYNODIAN-DEPOT-1440663828.pdf.
- ↑ "Gynodian Depoty". http://www.sukl.cz/download/pil/PI23959.pdf.
- ↑ Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. 6 December 2012. pp. 146–. ISBN 978-94-009-8195-9. https://books.google.com/books?id=7IrpCAAAQBAJ&pg=PA146.
- ↑ Geriatrics 3: Gynecology · Orthopaedics · Anesthesiology · Surgery · Otorhinolaryngology · Ophthalmology · Dermatology. Springer Science & Business Media. 6 December 2012. pp. 6–. ISBN 978-3-642-68976-5. https://books.google.com/books?id=vwzpCAAAQBAJ&pg=PA6.
- ↑ The Management of the Menopause & Post-Menopausal Years: The Proceedings of the International Symposium held in London 24–26 November 1975 Arranged by the Institute of Obstetrics and Gynaecology, The University of London. Springer Science & Business Media. 6 December 2012. pp. 395–. ISBN 978-94-011-6165-7. https://books.google.com/books?id=WT3sCAAAQBAJ&pg=PA395.
- ↑ Androgens in Health and Disease. Springer Science & Business Media. 27 May 2003. pp. 277–. ISBN 978-1-59259-388-0. https://books.google.com/books?id=vDcBCAAAQBAJ&pg=PA277.
- ↑ "The effects of hormone substitution in depot form on the uterus in a group of 50 perimenopausal women--a vaginosonographic study". Maturitas 21 (3): 221–225. April 1995. doi:10.1016/0378-5122(94)00893-c. PMID 7616871.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 11.7 11.8 11.9 "Gynodian Depot". https://www.drugs.com/international/gynodian-depot.html.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 Muller (19 June 1998). European Drug Index: European Drug Registrations, Fourth Edition. CRC Press. pp. 566–. ISBN 978-3-7692-2114-5. https://books.google.com/books?id=2HBPHmclMWIC&pg=PA566.
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 13.6 13.7 13.8 Pharmaceutical Substances, 5th Edition, 2009: Syntheses, Patents and Applications of the most relevant APIs. Thieme. 14 May 2014. pp. 1172–1174, 2441–2442. ISBN 978-3-13-179525-0. https://books.google.com/books?id=fO2IAwAAQBAJ&pg=PT2441.
- ↑ Dietary Supplements: Toxicology and Clinical Pharmacology. Springer Science & Business Media. 10 December 2002. pp. 123–147. ISBN 978-1-59259-303-3. https://books.google.com/books?id=vuqPBAAAQBAJ&pg=PA123.
- ↑ 15.0 15.1 "Novel mechanisms for DHEA action". Journal of Molecular Endocrinology 56 (3): R139–R155. April 2016. doi:10.1530/JME-16-0013. PMID 26908835.
- ↑ 16.0 16.1 16.2 16.3 16.4 16.5 16.6 "[Experience with a new hormone combination for menopausal disorders]" (in de). Medizinische Klinik 67 (11): 382–386. March 1972. PMID 4259772. "A new hormone combination for menopausal complaints. Since the treatment of menopausal complaints with estrogens as well as with the combination of estrogens and androgens causes undesired side effects such as bleeding, mammary changes and masculinisation, dehydroepiandrosteron (DHEA), a precursor of testosteron, has been synthesised, which has only a low conversion rate to free testosteron and no masculinising effect. The substance has been tested in combination with estrogen (200 mg DHEA-enanthate and 4 mg estradiolvalerianate per 1 ml) in 266 women with menopausal complaints. The duration of treatment has been up to 6 years with an injection interval of 3 to 8 weeks. The therapeutic results were as good as with estrogen-androgen-combinations, but there was no masculinising effect. Changes of voice, hair and libido caused by pretreatment partly disappeared. Side effects [such] as acne, mastodynia, and sensation of repletion were of transitory nature. This preparation seems to be a true alternative to the traditional estrogen-androgen-combinations.".
- ↑ 17.0 17.1 Erfolgsfaktoren für das marktorientierte Management patentgeschützter Arzneimittel: eine Analyse der Produktwahrnehmung niedergelassener Vertragsärzte unter der Berücksichtigung unsicherer Therapieergebnisse. BoD – Books on Demand. February 2008. pp. 37,346. ISBN 978-3-936863-12-3. https://books.google.com/books?id=C9k9qsEyTaMC&pg=PA37.
- ↑ 18.0 18.1 18.2 18.3 18.4 William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 1208–. ISBN 978-0-8155-1856-3. https://books.google.com/books?id=_J2ti4EkYpkC&pg=PA1208.
- ↑ 19.0 19.1 19.2 19.3 19.4 19.5 19.6 19.7 https://www.micromedexsolutions.com/
- ↑ "Erfahrungen in der Behandlung klimakterischer Beschwerden mit Depot-Injektionen von Östradiolvalerianat-Dehydroepiandrosteronönanthat". Die Therapiewoche 30 (10): 1736–1742. 1980. ISSN 0040-5973. https://scholar.google.com/scholar?cluster=4876387720777135191. "A trial of estradiol valerianate-dehydroandrosterone oenantate (Gynodian-Depot) was conducted in 68 post-menopausal women. The treatment exerted a very favorable influence on the typical subjective disorders of the climacteric and on the atrophic alterations of the target organs. Owing to its estrogenic and dehydroepiandrosterone components, the compound also exerts a favorable psychotropic effect. It was tolerated well and caused no side effects of any significance.".
- ↑ "[Treatment of the climacteric symptom complex with a new combined hormone preparation]" (in de). Fortschritte der Medizin 94 (9): 524–527. March 1976. PMID 134967.
- ↑ "[Gynodian-depot in the treatment of castration-induced postmenopause]" (in hr). Jugoslavenska Ginekologija I Perinatologija 27 (1–2): 37–40. 1987. PMID 2960859.
- ↑ D. J. Portman, S. R. Goldstein & R. Kagan (2019) Treatment of moderate to severe dyspareunia with intravaginal prasterone therapy: a review, Climacteric, 22(1), 65-72, https://doi.org/10.1080/13697137.2018.1535583
- ↑ "Serum oestrone, oestradiol and oestriol concentrations in castrated women during intramuscular oestradiol valerate and oestradiolbenzoate-oestradiolphenylpropionate therapy". Maturitas 2 (1): 53–58. January 1980. doi:10.1016/0378-5122(80)90060-2. PMID 7402086.
- ↑ "Serum testosterone, FSH/LH and urinary excretion of estrogens and corticoids during treatment with an injectable, longacting estrogen-DHEA preparation". Acta Obstetricia et Gynecologica Scandinavica 58 (4): 385–388. 1979. doi:10.3109/00016347909154601. PMID 160742.
- ↑ The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 641–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA641.
- ↑ Dictionary of Marine Natural Products with CD-ROM. CRC Press. 19 September 2007. pp. 1075–. ISBN 978-0-8493-8217-8. https://books.google.com/books?id=w1bLBQAAQBAJ&pg=PA1075.
- ↑ Die Gynäkologie. Springer-Verlag. 27 November 2013. pp. 917–. ISBN 978-3-662-11496-4. https://books.google.com/books?id=1pwiBgAAQBAJ&pg=PA917.
{{Navbox
| name = Androgens and antiandrogens | title = Androgens and antiandrogens | state = collapsed | listclass = hlist | groupstyle = text-align:center;
| group1 = Androgens
(incl. AAS)
| list1 =
| group2 = Antiandrogens | list2 = {{Navbox|child | groupstyle = text-align:center; | groupwidth = 9em;
| group1 = AR antagonists | list1 =
- Steroidal: Abiraterone acetate
- Canrenone
- Chlormadinone acetate
- Cyproterone acetate
- Delmadinone acetate
- Dienogest
- Drospirenone
- Medrogestone
- Megestrol acetate
- Nomegestrol acetate
- Osaterone acetate
- Oxendolone
- Potassium canrenoate
- Spironolactone
- Nonsteroidal: Apalutamide
- Bicalutamide
- Cimetidine
- Darolutamide
- Enzalutamide
- Flutamide
- Ketoconazole
- Nilutamide
- Seviteronel†
- Topilutamide (fluridil)
| group2 = Steroidogenesis| list2 =
inhibitors
| 5α-Reductase | |
|---|---|
| Others |
| group3 = Antigonadotropins | list3 =
- D2 receptor antagonists (prolactin releasers) (e.g., domperidone, metoclopramide, risperidone, haloperidol, chlorpromazine, sulpiride)
- Estrogens (e.g., bifluranol, [[diethylstilbestrol, estradiol, estradiol esters, ethinylestradiol, ethinylestradiol sulfonate, paroxypropione)
- GnRH agonists (e.g., leuprorelin)
- GnRH antagonists (e.g., cetrorelix)
- Progestogens (incl., chlormadinone acetate, [[cyproterone acetate, hydroxyprogesterone caproate, gestonorone caproate, [[Chemistry:Medroxyprogesterone medroxyprogesterone acetate, Chemistry:Megestrol acetate|megestrol acetate]])
| group4 = Others | list4 =
- Androstenedione immunogens: Androvax (androstenedione albumin)
- Ovandrotone albumin (Fecundin)
}}
| liststyle = background:#DDDDFF;| list3 =
- #WHO-EM
- ‡Withdrawn from market
- Clinical trials:
- †Phase III
- §Never to phase III
- See also
- Androgen receptor modulators
- Estrogens and antiestrogens
- Progestogens and antiprogestogens
- List of androgens/anabolic steroids
}}
 |