Biology:α-Methyltryptamine
 | |
 | |
| Clinical data | |
|---|---|
| Other names | Indopan; α-Methyl-3-indoleethanamine; 3-(2-Aminopropyl)indole; IT-290, IT-403, U-14,164E, 3-IT[1] |
| Routes of administration | Oral, Insufflation, Rectal, Smoked, IM, IV[1] |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
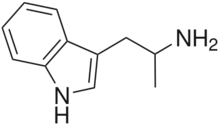
| Formula | C11H14N2 |
| Molar mass | 174.247 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
α-Methyltryptamine (abbreviated as αMT, AMT) is a psychedelic, stimulant, and entactogen drug of the tryptamine class.[2][3] It was originally developed as an antidepressant by chemists at Upjohn in the 1960s,[4] and was used briefly as an antidepressant in Russia under the trade name Indopan before being discontinued.[5][6][7]
Chemistry
αMT is a tryptamine with a methyl substituent at the alpha carbon. This alpha substitution makes it a relatively poor substrate for monoamine oxidase A, thereby prolonging αMT's half-life, allowing it to reach the brain and enter the central nervous system. Its chemical relation to tryptamine is analogous to that of amphetamine to phenethylamine, amphetamine being α-methylphenethylamine. αMT is closely related to the neurotransmitter serotonin (5-hydroxytryptamine) which partially explains its mechanism of action.
Synthesis
The synthesis of αMT can be accomplished through several different routes, the two most widely known being the Nitroaldol Condensation between Indole-3-carboxaldehyde and nitroethane under ammonium acetate catalysis which produces 1-(3-indolyl)-2-nitropropene-1 which can then be reduced using a reducing agent like lithium aluminum hydride[8] It's also hypothesized to be able to be reduced using Copper(II) chloride and Sodium borohydride, or the condensation between indole-3-acetone and Hydroxylamine [citation needed], followed by reduction of the obtained ketoxime with lithium aluminum hydride.[9]
Pharmacology
αMT acts as a relatively balanced reuptake inhibitor and releasing agent of the main three monoamines; serotonin, norepinephrine, and dopamine,[10] and as a non-selective serotonin receptor agonist.[11]
MAOI activity
αMT has been shown as a reversible inhibitor of the enzyme monoamine oxidase (MAO) in-vitro[12] and in-vivo.[13]
In rats the potency of αMT as an MAO-A inhibitor in the brain was approximately equal to that of harmaline at equimolar doses.[note 1] Dextroamphetamine did not enhance the 5-hydroxytryptophan-induced rise of serotonin at any level.[14]
Metabolism
2-Oxo-αMT, 6-hydroxy-αMT,[15] 7-hydroxy-αMT and 1′-hydroxy-αMT were detected as metabolites of αMT in male Wistar rats .[16]
Dosage and effects
Under the trade name Indopan, 5-10 milligrams were used for an antidepressant effect.
With 20–30 milligrams, euphoria, empathy, and psychedelic effects become apparent and can last as long as 12 hours.[17] A dose exceeding 40 mg is generally considered strong. In rare cases or extreme doses, the duration of effects might exceed 24 hours. Users report that αMT in freebase form is smoked, with doses between and 2 and 5 milligrams.[1][unreliable source?][2]
Neurologic side effects of αMT include agitation, restlessness, confusion, and lethargy. Physical manifestations including vomiting, mydriasis (pupillary dilation), jaw clenching, tachycardia, salivation, diaphoresis (sweating), and elevations in blood pressure, temperature, and respiratory rate.[18]
Side effects self-reported by recreational users include anxiety, muscle tension, jaw tightness, pupil dilation, tachycardia, headaches, nausea, and vomiting, as well as psychedelic effects including visual hallucinations and an altered state of mind.[2][19]
Legality
Australia
The 5-Methoxy analogue, 5-MeO-αMT is schedule 9 in Australia and αMT would be controlled as an analogue of this.[20]
China
As of October 2015 αMT is a controlled substance in China.[21]
Denmark
In Denmark (2010), the Danish Minister for the Interior and Health placed αMT to their lists of controlled substances (List B).[22]
Canada
Canada has no mention of αMT in the Controlled Drugs and Substances Act.[23]
Germany
αMT is listed under the Narcotics Act in schedule 1 (narcotics not eligible for trade and medical prescriptions) in Germany.[22]
Austria
αMT is placed under Austrian law (NPSG) Group 6.[22]
Hungary
αMT was controlled on the Schedule C list in Hungary in 2013.[22]
Slovakia
αMT was placed in 2013 on the List of Hazardous Substances in Annex, § 2 in Slovakia.[22]
Slovenia
αMT appeared on the Decree on Classification of Illicit Drugs in Slovenia (2013).[22]
Lithuania
In Lithuania (2012), αMT is controlled as a tryptamine derivative put under control in the 1st list of Narcotic Drugs and Psychotropic Substances which use is prohibited for medical purposes.[22]
Spain
αMT is legal in Spain.[24]
Sweden
Sveriges riksdags health ministry Statens folkhälsoinstitut classified αMT as "health hazard" under the act Lagen om förbud mot vissa hälsofarliga varor (translated Act on the Prohibition of Certain Goods Dangerous to Health) as of Mar 1, 2005, in their regulation SFS 2005:26 listed as alfa-metyltryptamin (AMT), making it illegal to sell or possess.[25]
United Kingdom
αMT was made illegal in the United Kingdom as of 7 January 2015, along with 5-MeO-DALT.[26] This was following the events of 10 June 2014 when the Advisory Council on the Misuse of Drugs recommended that αMT be scheduled as a class A drug by updating the blanket ban clause on tryptamines.[27]
United States
The Drug Enforcement Administration (DEA) placed αMT temporarily in schedule I of the Controlled Substances Act (CSA) on April 4, 2003, pursuant to the temporary scheduling provisions of the CSA (68 FR16427). On September 29, 2004, αMT was permanently controlled as a schedule I substance under the CSA (69FR 58050).[28]
Finland
AMT, alfa-methyltryptamine is a controlled drug in Finland[29]
Reported deaths
αMT is capable of causing life-threatening side-effects including hyperthermia, hypertension, and tachycardia.[18][30] Fatalities have been reported in association with high doses or concomitant use of other drugs.[31] Fatalities verified with toxicology and autopsy include those of a 22-year-old man in Miami-Dade county and a British teenager, both who died after consuming 1 g of αMT.[32][18]
See also
- 4-Methyl-αMT
- 5-Fluoro-αMT
- 5-MeO-αMT
- α-Ethyltryptamine
- α,N-DMT
- α,N,N-TMT
- α-Methyl-5-HT
Notes
References
- ↑ 1.0 1.1 1.2 "Erowid AMT Vault : FAQ by Dialtonez". http://www.erowid.org/chemicals/amt/amt_faq1.shtml.
- ↑ 2.0 2.1 2.2 "Erowid Online Books : "TIHKAL" - #48 a-MT". http://www.erowid.org/library/books_online/tihkal/tihkal48.shtml.
- ↑ "Erowid AMT (alpha-methyltryptamine) Vault". http://www.erowid.org/chemicals/amt/amt.shtml.
- ↑ Szmuszkovicz Jacob, "Method of Treating Mental Depression", US patent Patent 3296072, published 1967-01-03, assigned to Upjohn Co
- ↑ Medical Toxicology of Drug Abuse: Synthesized Chemicals and Psychoactive Plants. John Wiley & Sons. 20 March 2012. pp. 196–. ISBN 978-0-471-72760-6. https://books.google.com/books?id=OWFiVaDZnkQC&pg=PA196.
- ↑ Handbook of Psychopharmacology: Volume 14 Affective Disorders: Drug Actions in Animals and Man. Springer Science & Business Media. 11 November 2013. pp. 132–. ISBN 978-1-4613-4045-4. https://books.google.com/books?id=ogXrBwAAQBAJ&pg=PA132.
- ↑ Biological Research on Addiction: Comprehensive Addictive Behaviors and Disorders. Academic Press. 17 May 2013. pp. 632–. ISBN 978-0-12-398360-2. https://books.google.com/books?id=ZUeCgcrNjOUC&pg=PA632.
- ↑ "TheHive". https://chemistry.mdma.ch/hiveboard/rhodium/it-290.html.
- ↑ "TIHKAL". https://erowid.org/library/books_online/tihkal/tihkal48.shtml.
- ↑ "The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain". European Journal of Pharmacology 559 (2–3): 132–137. March 2007. doi:10.1016/j.ejphar.2006.11.075. PMID 17223101.
- ↑ "In vitro screening of psychoactive drugs by [(35)S]GTPgammaS binding in rat brain membranes". Biological & Pharmaceutical Bulletin 30 (12): 2328–2333. December 2007. doi:10.1248/bpb.30.2328. PMID 18057721.
- ↑ "Studies of monoamine oxidase and semicarbazide-sensitive amine oxidase. II. Inhibition by alpha-methylated substrate-analogue monoamines, alpha-methyltryptamine, alpha-methylbenzylamine and two enantiomers of alpha-methylbenzylamine". Japanese Journal of Pharmacology 41 (2): 191–197. June 1986. doi:10.1254/jjp.41.191. PMID 3747266.
- ↑ "The effect of three tryptamine derivatives on serotonin metabolism in vitro and in vivo". The Journal of Pharmacology and Experimental Therapeutics 127: 110–115. October 1959. PMID 13851725. http://jpet.aspetjournals.org/content/127/2/110.short.
- ↑ 14.0 14.1 "Effect of alpha-alkylated tryptamine derivatives on 5-hydroxytryptamine metabolism in vivo". British Journal of Pharmacology and Chemotherapy 19 (1): 161–167. August 1962. doi:10.1111/j.1476-5381.1962.tb01437.x. PMID 13898151.
- ↑ "6-Hydroxylation: an important metabolic route for alpha-methyltryptamine". Experientia 17 (2): 76–77. February 1961. doi:10.1007/BF02171429. PMID 13774483.
- ↑ "In vivo metabolism of alpha-methyltryptamine in rats: identification of urinary metabolites". Xenobiotica; the Fate of Foreign Compounds in Biological Systems 38 (12): 1476–1486. December 2008. doi:10.1080/00498250802491654. PMID 18982537.
- ↑ "Psychoactive properties of alpha-methyltryptamine: analysis from self reports of users". Journal of Psychoactive Drugs 44 (3): 274–276. 2012. doi:10.1080/02791072.2012.704592. PMID 23061328.
- ↑ 18.0 18.1 18.2 "Fatality due to acute alpha-methyltryptamine intoxication". Journal of Analytical Toxicology 29 (5): 394–397. July–August 2005. doi:10.1093/jat/29.5.394. PMID 16105268.
- ↑ "Erowid AMT Vault : Effects". http://www.erowid.org/chemicals/amt/amt_effects.shtml.
- ↑ "Erowid 5-MeO-AMT Vault : Legal Status". http://www.erowid.org/chemicals/5meo_amt/5meo_amt_law.shtml.
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in zh). China Food and Drug Administration. 27 September 2015. http://www.sfda.gov.cn/WS01/CL0056/130753.html.
- ↑ 22.0 22.1 22.2 22.3 22.4 22.5 22.6 "Reference at www.who.int". https://www.who.int/medicines/areas/quality_safety/4_20_Review.pdf.
- ↑ "CSDA". http://isomerdesign.com/Cdsa/schedule.php?structure=C.
- ↑ "Medicamentos de Uso Humano - Estupefacientes y Psicótropos". https://www.aemps.gob.es/medicamentosUsoHumano/estupefacientesPsicotropos/home.htm.
- ↑ "Förordning om ändring i förordningen (1999:58) om förbud mot vissa hälsofarliga varor;" (in sv), Lagen om förbud mot vissa hälsofarliga varor - SFS 2005:26, February 2005, http://www.notisum.se/rnp/sls/sfs/20050026.pdf, retrieved 2013-10-07
- ↑ "The Misuse of Drugs (Amendment No. 3) (England, Wales and Scotland) Regulations 2014". http://www.legislation.gov.uk/uksi/2014/3277/introduction/made/data.htm.
- ↑ ACMD (10 June 2014). "Update of the Generic Definition for Tryptamines". UK Home Office. p. 12. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/318693/UpdateGenericDefinitionTryptamines.pdf.
- ↑ "Alpha-methyltryptamine". DEA Office of Diversion Control. April 2013. http://www.deadiversion.usdoj.gov/drug_chem_info/amt.pdf.
- ↑ "Reference at www.finlex.fi". http://www.finlex.fi/data/sdliite/liite/6194.pdf.
- ↑ "Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity". British Journal of Anaesthesia 95 (4): 434–441. October 2005. doi:10.1093/bja/aei210. PMID 16051647. "drugs such as MDMA, ecstasy (3,4-methylenedioxymethamphetamine), if combined with MAOIs (including moclobemide) do also cause fatalities because they act as serotonin releasers"
- ↑ "Call for ban on drug after reveller's death". Western Gazette. March 22, 2012. http://www.thisissomerset.co.uk/Torch-carriers-miles-home/story-15588409-detail/story.html#axzz2glqAXLdX.
- ↑ "Southampton 'legal high' death deemed 'accidental'". BBC News. 2013-11-12. https://www.bbc.co.uk/news/uk-england-hampshire-24915409.
External links
 |

