Chemistry:Apigenin
| |
| |
| Names | |
|---|---|
| IUPAC name
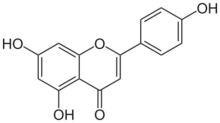
4′,5,7-Trihydroxyflavone
| |
| Systematic IUPAC name
5,7-Dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one | |
| Other names
Apigenine; Chamomile; Apigenol; Spigenin; Versulin; C.I. Natural Yellow 1
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H10O5 | |
| Molar mass | 270.240 g·mol−1 |
| Appearance | Yellow crystalline solid |
| Melting point | 345 to 350 °C (653 to 662 °F; 618 to 623 K) |
| UV-vis (λmax) | 267, 296sh, 336 nm in methanol[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Apigenin (4′,5,7-trihydroxyflavone), found in many plants, is a natural product belonging to the flavone class that is the aglycone of several naturally occurring glycosides. It is a yellow crystalline solid that has been used to dye wool.
Sources in nature
Apigenin is found in many fruits and vegetables, but parsley, celery, celeriac, and chamomile tea are the most common sources.[3] Apigenin is particularly abundant in the flowers of chamomile plants, constituting 68% of total flavonoids.[4] Dried parsley can contain about 45 mg apigenin/gram of the herb, and dried chamomile flower about 3–5 mg/gram.[5] The apigenin content of fresh parsley is reportedly 215.5 mg/100 grams, which is much higher than the next highest food source, green celery hearts providing 19.1 mg/100 grams.[6]
Pharmacology
Apigenin competitively binds to the benzodiazepine site on GABAA receptors.[7] There exist conflicting findings regarding how apigenin interacts with this site.[8][9]
Biosynthesis
Apigenin is biosynthetically derived from the general phenylpropanoid pathway and the flavone synthesis pathway.[10] The phenylpropanoid pathway starts from the aromatic amino acids L-phenylalanine or L-tyrosine, both products of the Shikimate pathway.[11] When starting from L-phenylalanine, first the amino acid is non-oxidatively deaminated by phenylalanine ammonia lyase (PAL) to make cinnamate, followed by oxidation at the para position by cinnamate 4-hydroxylase (C4H) to produce p-coumarate. As L-tyrosine is already oxidized at the para position, it skips this oxidation and is simply deaminated by tyrosine ammonia lyase (TAL) to arrive at p-coumarate.[12] To complete the general phenylpropanoid pathway, 4-coumarate CoA ligase (4CL) substitutes coenzyme A (CoA) at the carboxy group of p-coumarate. Entering the flavone synthesis pathway, the type III polyketide synthase enzyme chalcone synthase (CHS) uses consecutive condensations of three equivalents of malonyl CoA followed by aromatization to convert p-coumaroyl-CoA to chalcone.[13] Chalcone isomerase (CHI) then isomerizes the product to close the pyrone ring to make naringenin. Finally, a flavanone synthase (FNS) enzyme oxidizes naringenin to apigenin.[14] Two types of FNS have previously been described; FNS I, a soluble enzyme that uses 2-oxogluturate, Fe2+, and ascorbate as cofactors and FNS II, a membrane bound, NADPH dependent cytochrome p450 monooxygenase.[15]
Glycosides
The naturally occurring glycosides formed by the combination of apigenin with sugars include:
- Apiin (apigenin 7-O-apioglucoside), isolated from parsley[16] and celery
- Apigetrin (apigenin 7-glucoside), found in dandelion coffee
- Vitexin (apigenin 8-C-glucoside)
- Isovitexin (apigenin 6-C-glucoside)
- Rhoifolin (apigenin 7-O-neohesperidoside)
- Schaftoside (apigenin 6-C-glucoside 8-C-arabinoside)
In diet
Some foods contain relatively high amounts of apigenin:[17]
| Product | Apigenin (milligrams per 100 grams) |
|---|---|
| Parsley | 4504 |
| Celery hearts, green | 19 |
| Rutabagas, raw | 4 |
See also
References
- ↑ Merck Index, 11th Edition, 763.
- ↑ The Systematic Identification of Flavonoids. Mabry et al, 1970, page 81
- ↑ The compound in the Mediterranean diet that makes cancer cells 'mortal' Emily Caldwell, Medical Express, May 20, 2013.
- ↑ "Curcumin and Apigenin - novel and promising therapeutics against chronic neuroinflammation in Alzheimer's disease". Neural Regeneration Research 10 (8): 1181–5. August 2015. doi:10.4103/1673-5374.162686. PMID 26487830.
- ↑ "Plant flavone apigenin: An emerging anticancer agent". Current Pharmacology Reports 3 (6): 423–446. 2017. doi:10.1007/s40495-017-0113-2. PMID 29399439.
- ↑ Delage, PhD, Barbara (November 2015). "Flavonoids". Corvallis, Oregon: Linus Pauling Institute, Oregon State University. http://lpi.oregonstate.edu/mic/dietary-factors/phytochemicals/flavonoids.
- ↑ Viola, H.; Wasowski, C.; Levi de Stein, M.; Wolfman, C.; Silveira, R.; Dajas, F.; Medina, J. H.; Paladini, A. C. (June 1995). "Apigenin, a component of Matricaria recutita flowers, is a central benzodiazepine receptors-ligand with anxiolytic effects". Planta Medica 61 (3): 213–216. doi:10.1055/s-2006-958058. ISSN 0032-0943. PMID 7617761. https://pubmed.ncbi.nlm.nih.gov/7617761/.
- ↑ Dekermendjian, K.; Kahnberg, P.; Witt, M. R.; Sterner, O.; Nielsen, M.; Liljefors, T. (1999-10-21). "Structure-activity relationships and molecular modeling analysis of flavonoids binding to the benzodiazepine site of the rat brain GABA(A) receptor complex". Journal of Medicinal Chemistry 42 (21): 4343–4350. doi:10.1021/jm991010h. ISSN 0022-2623. PMID 10543878. https://pubmed.ncbi.nlm.nih.gov/10543878/.
- ↑ Avallone, R.; Zanoli, P.; Puia, G.; Kleinschnitz, M.; Schreier, P.; Baraldi, M. (2000-06-01). "Pharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomilla". Biochemical Pharmacology 59 (11): 1387–1394. doi:10.1016/s0006-2952(00)00264-1. ISSN 0006-2952. PMID 10751547. https://pubmed.ncbi.nlm.nih.gov/10751547/.
- ↑ Forkmann, G. (January 1991). "Flavonoids as Flower Pigments: The Formation of the Natural Spectrum and its Extension by Genetic Engineering" (in en). Plant Breeding 106 (1): 1–26. doi:10.1111/j.1439-0523.1991.tb00474.x. ISSN 0179-9541.
- ↑ "The shikimate pathway as an entry to aromatic secondary metabolism". Plant Physiology 107 (1): 7–12. January 1995. doi:10.1104/pp.107.1.7. PMID 7870841.
- ↑ "Biosynthesis of Two Flavones, Apigenin and Genkwanin, in Escherichia coli". Journal of Microbiology and Biotechnology 25 (9): 1442–8. September 2015. doi:10.4014/jmb.1503.03011. PMID 25975614.
- ↑ "The chalcone synthase superfamily of type III polyketide synthases". Natural Product Reports 20 (1): 79–110. February 2003. doi:10.1039/b100917f. PMID 12636085.
- ↑ "Cloning of parsley flavone synthase I". Phytochemistry 58 (1): 43–6. September 2001. doi:10.1016/S0031-9422(01)00191-1. PMID 11524111.
- ↑ "Investigation of two distinct flavone synthases for plant-specific flavone biosynthesis in Saccharomyces cerevisiae". Applied and Environmental Microbiology 71 (12): 8241–8. December 2005. doi:10.1128/AEM.71.12.8241-8248.2005. PMID 16332809. Bibcode: 2005ApEnM..71.8241L.
- ↑ "Bioavailability of apigenin from apiin-rich parsley in humans". Annals of Nutrition & Metabolism 50 (3): 167–72. 2006. doi:10.1159/000090736. PMID 16407641. https://nbn-resolving.org/urn:nbn:de:bvb:384-opus4-857673.
- ↑ USDA Database for the Flavonoid Content of Selected Foods, Release 3 (2011)
 |

